CORPORATE OVERVIEW
JANUARY 2009
This presentation does not constitute an offering of any securities for sale or a solicitation
of offers to purchase securities. The Company's securities may not be offered or sold
absent registration
under applicable securities laws or an exemption from registration. Any
public offering of the securities of the Company will be made by means of a prospectus
that will contain detailed information about the offering and the Company.

Safe Harbor
Under the Private Securities Litigation Reform Act of 1995: This release includes
forward-looking statements that reflect ONI BioPharma's current views with respect to
future events and financial performance.
These forward-looking statements are based
on management's beliefs and assumptions and information currently available. The
words "believe," "expect," "anticipate," "intend," "estimate," "project" and similar
expressions that do not relate solely
to historical matters identify forward-looking
statements. Investors should be cautious in relying on forward-looking statements
because they are subject to a variety of risks, uncertainties, and other factors that could
cause actual results to
differ materially from those expressed in any such forward-
looking statements. These factors include: future costs associated with any potential
listing sponsor, those factors set forth in our most recently filed annual report on Form
10-KSB and
quarterly report on Form 10-Q, and other factors detailed from time to time
in filings with the Securities and Exchange Commission. We expressly disclaim any
responsibility to update forward-looking
statements.
2
ONI BioPharma is a multi-faceted biopharmaceutical
company with operations in four divisions:
Consumer Healthcare
Diagnostics
Replacement Therapy
Antibiotics
ONI is commercializing its first consumer products:
- Probiora3TM
, the first full-care oral probiotic that promotes healthy teeth and gums and whitens teeth
and freshens breath.
- LPT3-04TM
for weight loss is in clinical trials and, if successful, should be ready for marketing in 2009.
ONI expects to generate revenue from consumer products in 2009 both in the US and internationally.
Proceeds will support the Company’s diagnostic, replacement therapy, and antibiotic divisions.
Patents, strategic sales channels, and international partners will support ONI’s growth. ONI is developing
commercial and research facilities in Mexico and France to support its global strategy. ONI was
founded
in 1996 by Dr. Jeffrey Hillman, a recognized expert in molecular genetics. The Company is based in
Alachua, Florida.
Corporate Mission
3
Financial Highlights
4
OTCBB: ORNI
NYSE Alternext Paris: ALONI
Industry: Biopharmaceutical
Equity Cap: $13.03M (US)
As of 1/2/09
Volume:
33,843
Shares Outstanding: 38.3M
Float:
21.6M
Long-term debt: 0
Founded: 1996

Four Product Divisions
Consumer Healthcare
DPOLT/Antibiotics Platform
Diagnostics
Replacement Therapy
ONI’s science has developed into four distinct categories.
Technologies complement one another. ONI intends to use revenue
generated by initial consumer products to support research and
development
of longer-term therapies.
5

Technology Overview
Mixture of 3 natural beneficial oral
bacteria for
Maintaining dental (tooth) health
Maintaining periodontal (gum) health
Whitening teeth
Promoting fresh breath
Probiora3™

Safety and activity established in animals
Novel MOA - selectively kills white fat cells
Strong evidence for safety in humans
Clinical trial in Q2
LPT3-04™ : Weight Loss Agent
Our lead antibiotic
Novel mechanism of action
Safe and very active in pilot studies
MRSA
Most other gram positive infections
Mtb, both growing and dormant
No evidence for development of resistance
Hard to make by conventional fermentation
methods
DPOLT
MU1140™ - A Focus Techno
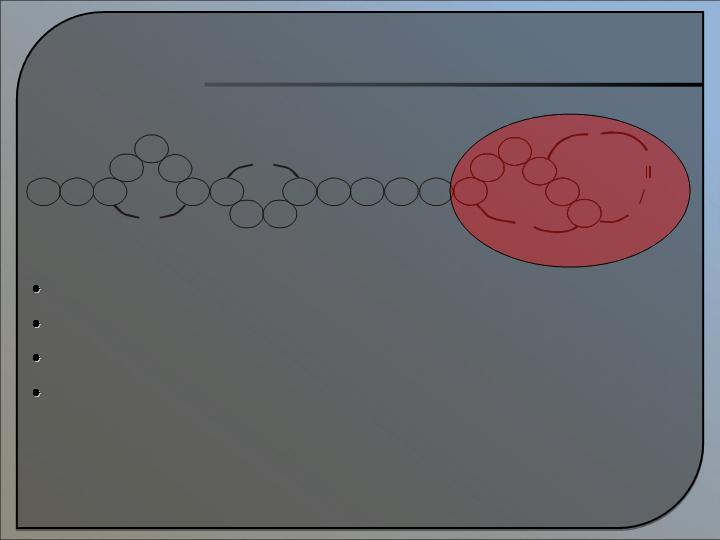
Platform for organic synthesis of MU1140
Proof of principle completed
Cost effective GMP production
Can make analogues of MU1140 and the other 50 known
lantibiotics
ala
abu
pro
gly
ala
ala
dhb
gly
ala
phe
asn
ala
tyr
ala
S
NH
CH
CH
S
gly
ala
phe
asn
ala
tyr
ala
S
NH
CH
CH
S
S
S
phe
lys
ala
trp
dha
leu
arg
A
D
C
B
DPOLT™ - A Focus
Fast, sensitive, superior method for identification of targets for
new diagnostics, vaccines and drugs
Human infections
Plant infections
Cancers
Also applicable to various other problems such as
bioscavenging and biofouling
IVIAT / CMAT – A Focus

Genetically modified Streptococcus mutans
One-time treatment for lifelong protection against most tooth
decay
SMaRT

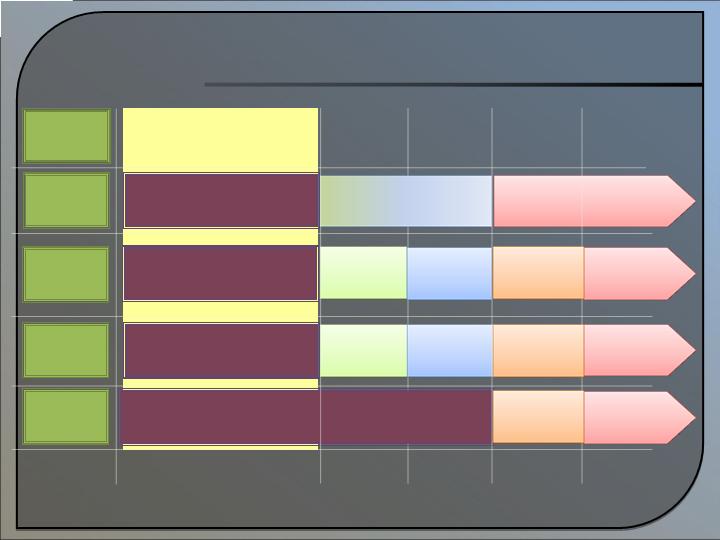
Strategy and Forecasted
Calendar of Development
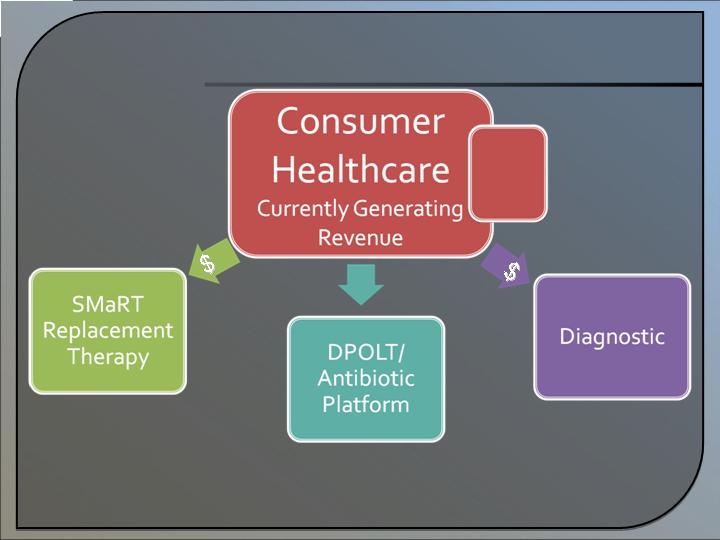
Revenue Model
15
$
$
* Consumer Health revenue is expected to support the growth of other business units.
Consumer
Healthcare
2010
Diagnostic
Replacement
Therapy
15 projects completed
Second Phase I clinical trial
DPOLT/
Antibiotic
Platform
Pro Biora3™
LPT3-04™
Commercially Available:
EvoraPet™,
EvoraKids™,
EvoraPLUS
™
Regulatory approval &
marketing Evora & LPT3-04™
in Europe, Asia, South America
2009
2012
5 molecules made
MU1140 IND filed
Market first
antibiotic,
MU1140
15 proj.
completed
Three 510(k)s
filed
Commercially
Available
2011
2013
Market
SMaRT
16
Phase II/III clinical trial
5 molecules
made
1 IND filed
5 molecules
made
1 IND filed
5 molecules
made
1 IND filed
15 proj.
completed
Three 510(k)s
filed
15 proj.
completed
Three 510(k)s
filed
15 proj.
completed
Three 510(k)s
filed
Projected Summary Timeline
2009-2013
Consumer Healthcare
Benefits/Characteristics:
GRAS – Generally Recognized as Safe status (vs FDA approval)
Significantly reduces regulatory costs
Fast to market
Faster generation of gross and net revenues
Lower capital requirements
Marketed in US and internationally (Mexico in early 2009)
Sales and marketing arrangements in place
Human and companion animal applications
2009
2010
Oral Health
ProBiora3™– Finish identity work & mass
market companion pet product
Commence marketing upon granting of
regulatory exemption status – non-US
Weight Loss
Finish all clinical trials
Begin selling LPT3-04™
Begin seeking regulatory exemptions in
non-US markets (Europe, Asia & South
America)
Begin global marketing
17
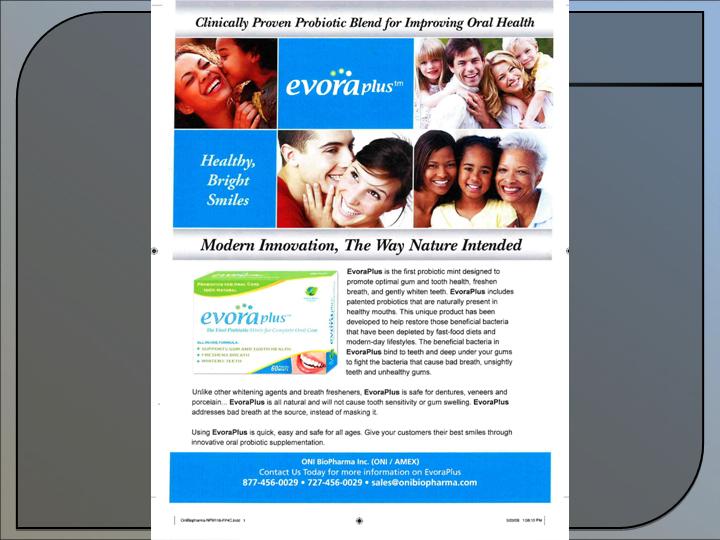
ProBiora3™ - Probiotic mint to promote gum and dental health.
LPT3-04™ - Natural weight loss compound to
kill white fat cells.

Consumer Product Manufacturers
Direct Selling Companies
Dental Product Companies
Contract Private Label Companies
Natural Product Companies
Pet Product Companies
Mass Retail
Internet
Independent Health Food Stores
Independent Pharmacies
Dental Offices
Direct Response
Direct Mail
Government
Media and Distribution Strategy

EvoraPlus Activity
19
Existing Internet & Dental
Meetings With 50+ Mass Retailers

Confidential
Probiotics Market
20
Probiotics: live microorganisms, which when administered in adequate
amounts, confer a health benefit on the host.
Generally associated with the gut health, though they have been
promoted for urogenital health, irritable bowel syndrome, bladder
cancer and to boost the immune system.
Only 40 probiotic products in the US market in 2005. In last three years
more than 250 new products were launched.
Yakult (Japan) is credited with beginning the probiotics revolution and
sales of its lead product in 2007 were estimated at $2.5 billion.
In addition to Yakult, major players in this market include Danone,
Nestle, Danisco, DSM and Proctor & Gamble.
Americans spent close to $80 billion on oral healthcare in 2007, with over
$5 billion in OTC products.
ONI BioPharma has the FIRST probiotics product that represents an
entry into the total oral health care market.
DPOLT/Antibiotics Platform
Benefits/Characteristics:
Overcomes 80+ years of previous failures
Predicted to lead to new antibiotics with novel mechanisms of action
Potential substantial antibiotic pipeline
Replaces current antibiotic treatments that are failing
2009*
2010*
2011*
2012-2013*
MU1140 IND filed and 5
new molecules made
5 new molecules made
and 1 new IND filed
5 new molecules made
and 1 new IND filed
Market MU1140, 5 new
molecules made and 1
new IND filed
21
DPOLT™ - Novel organic chemistry synthesis platform enabling large-scale,
cost-effective production of clinical grade MU1140 and 50
other known lantibiotics.
* EXPECTED MILESTONES
Diagnostics
2009*
2010*
2011*
2012*
2013*
15 proj. completed
15 proj. completed
Three 510(k)s filed
15 proj. completed
Three 510(k)s filed
15 proj. completed
Three 510(k)s filed
15 proj. completed
Three 510(k)s filed
22
PIVIAT™ - Rapid and sensitive identification of novel targets for use in diagnosis and
treatment
of human infectious diseases.
PCMAT™ - Rapid and sensitive identification of novel targets for diagnostics and therapeutic
strategies that address an extraordinary
range of medical, agricultural, and industrial applications.
* EXPECTED MILESTONES

Replacement Therapy
2009*
2010*
2012*
Second Phase I clinical trial
Phase II/III FDA clinical trials
Market SMaRT™
Benefits/Characteristics:
Efficiency
Mexico trials = 50% cost savings (vs. US)
Regulatory Speed
ONI Mexico (subsidiary) should attain faster regulatory access within Mexican government
Reduced “red-tape” associated with government clearance to clinical trials
Preventative medicines/treatments typically receive faster acceptance and clearance by
governments worldwide
23
SMaRT™ - a single, painless application of a genetically modified bacterial strain for
tooth surfaces to protect against tooth decay.
* EXPECTED MILESTONES

Timeline
Oragenics
(ONI BioPharma)
Founded by
Jeffrey Hillman
2008
1996
Completes Initial
public offering (IPO)
2005
2009
2003
2004
Obtains $9M
financing
Initiates Phase I studies for
tooth decay-replacement
therapy
Seeks patent to
treat obesity-
LPT3-04™
2006
Acquires iviGene
Corporation and
gene/biomarker
assets
Patent issued
for
MU 1140™
SMaRT Phase I
trials begin
2007
Receives European
patent for IVIAT™
biomarker
technology
Begin test
marketing
ProBiora3 tablets
Starts to do
business as ONI
BioPharma
David Hirsch joins
as COO & CFO
Launch of
ProBiora3™
Website
Successful
synthesis using
DPOLT
technology
Alternext
Paris listing
ONI Mexico
established
2010
ProBiora3™
approval &
marketing
globally
Replacement
therapy SMaRT
phase II
2011
2012
Diagnostic
projects &
licensing
continues
Market SMaRT
Market MU
1140
24
Projected
Confidential
Management
Stanley B. Stein, Chief Executive Officer, President and Director
25 years in securities in biotech & healthcare industry
Managing Director at Drexel Burnham Lambert, Inc.
Founded SRS Capital - boutique investment firm; specializing in healthcare
Principal investment banker in the creation of Fresenius Medical Care AG, the
largest renal care business in the world
Jeffry Hillman, DMD, PhD, Chief Scientific Officer and Director
Founded ONI to commercialize the fruits of 25 years of research at the Harvard-affiliated
Forsyth Institute in Boston and the University of Florida
Author of 125+ publications
Undergraduate training at the University of Chicago; DMD degree from Harvard School of
Dental Medicine; PhD from Harvard Medical School
David B. Hirsch, Chief Financial
Officer and Chief Operating Officer
Manager in the Restructuring Group at Deloitte and Touche
Associate at The Cottonwood Group, a venture capital firm in San Mateo, California
MBA from the Tepper School of Business at Carnegie Mellon University and a JD from
Drake University Law School
Licensed attorney in Florida and Indiana
Corporate
Structuring
Science
Legal/
Accounting
25
Confidential
Outside Directors –
Expertise in: Management , International Business, Academic Science & Media
Rick Welch, Chairman
President of Welch Business Solutions and Consulting, LLC in Tampa, Florida
Director and CFO for the following: Orthopedic Development Corporation, Albiorex, LLC, Medi-Spa's of
America, Inc, and Vision Twenty-One
Previously CFO/Executive Vice-President of Finance and Administration, for Sports and Recreation, Inc.
Derek Hennecke, Director
Independent Board member and expert in drug development
Founder and CEO of Xcelience, a formulation and clinical manufacturing contract research
company in Tampa,
Florida
25+ years in the international biotechnology; MDS Pharma Sciences, DSM (contract
manufacturing company) (NBD)
Boehringer Mannheim in NBD
Work experience in Europe, Egypt, Mexico, Canada, and USA
Kevin Sills, Director
Independent director Vice President of Pharmaceutical Development of King
Pharmaceuticals Research & Development
25+ years related experience, novel chemical formulations, clinical supplies management
Faculty experience at University of North Carolina: Center for Professional Advancent; President of NC
Pharmaceutical Discussion Group
Active member of the Licensing Executives Society and the American Assoc of Pharmaceutical
Scientists
Dr. Marc Siegel, Director
Clinical Associate Professor of Medicine at NYU School of Medicine
Medical Director of Doctor Radio with NYU and Sirius Satellite Radio
Fox News Medical Contributor
Columnist for the Los Angeles Times, a member of the Board of Contributors at USA Today, regular contributor to the
NY Post, and frequent contributor to the
Washington Post, the Wall Street Journal, and Newsday
26
Confidential
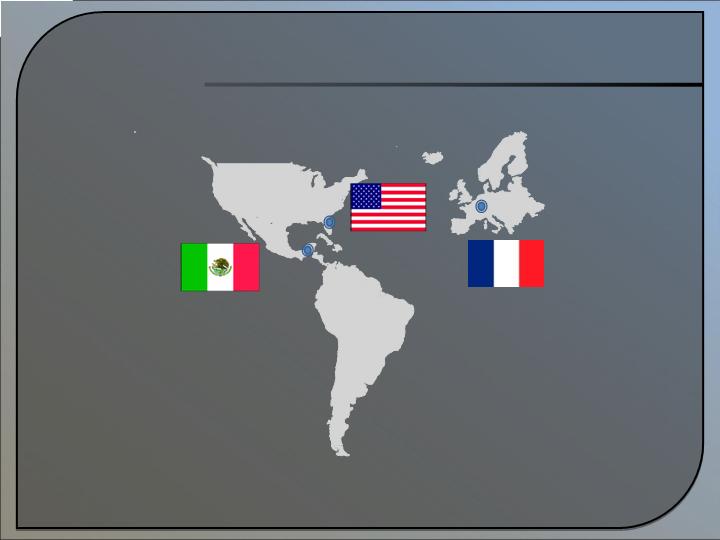
Global Expansion
27
Mexico
Subsidiary (ONI Biotec, Mexico)
launch - Q1 2009
France
Alternext Paris Listing –
Planned operations in European Union
USA – Alachua, FL
ONI Corporate Offices
est. 1996

An anchor point for the commercial conquest of Europe
Significant market : popularity of probiotics among European consumers
Central location
Strong scientific and industrial network
Attractive political environment
“Fast Path” to Listing
Direct listing (use of SEC filings for Information Document)
Tailored market place
Listing sponsor
Exposure as a listed company
Qualified investors (Small & Mid Caps / health care and biotech)
Inclusion in the NYSE Euronext Next Biotech index
Supporting growth in Europe
Why a Listing in Paris?

Summary

Confidential
Summary
Sales & near-term revenue generated from consumer product,
Probiora3™.
Facilities & business relations in the United States, Mexico,
South America and Europe.
Multiple strategies for getting consumer and biotech products to
market.
Targeting global healthcare segments where there is distinct
need and growing population.
Undiscovered by Wall Street.
30

Confidential
31
In the News
Probiotics - Topic Overview
What are Probiotics?
Why Probiotics?
Probiotics May Help People Taking Antibiotics
What health benefits do they offer?
What is important to know about Probiotics?
Confidential
Investor Contacts
32
Global Headquarters:
Stanley B. Stein
Chief Executive Officer
ONI BioPharma, Inc.
13700 Progress Blvd
Alachua, FL 32615
Ph: (386) 418-4018
sstein@onibiopharma.com
www.onibiopharma.com
Investor Contact:
Axelle Vuillermet
Pierre Laurent
8 place de la Madeleine
75008 Paris
Tél. : 01 44 71 94 94
avuillermet@newcap.fr
plaurent@newcap.fr