
Martin Handfield, MS, PhD SVP, Research 386 418 4018 ext 241 mhandfield@oragenics.com Oragenics, Inc. (NYSE: OGEN) 13700 Progress Blvd Alachua, FL 32615 www.oragenics.com Lantibiotics for the Treatment of Clostridium difficile Associated Disease Exhibit 99.1

Safe Harbor Statement Certain statements made in this presentation include forward-looking actions that Oragenics, Inc. (“Oragenics,” or the “Company”) anticipates based on certain assumptions. These statements are indicated by words such as “expect”, “anticipate”, “should” and similar words indicating uncertainty in facts, figures and outcomes. Such statements are made pursuant to the Safe Harbor Provisions of the Private Securities Litigation Reform Act of 1995. While Oragenics believes that the expectations reflected in such forward-looking statements are reasonable, it can give no assurance that such statements will prove to be correct. The risks associated with the Company are detailed in the Company’s various reports filed by the Company with the Securities and Exchange Commission.
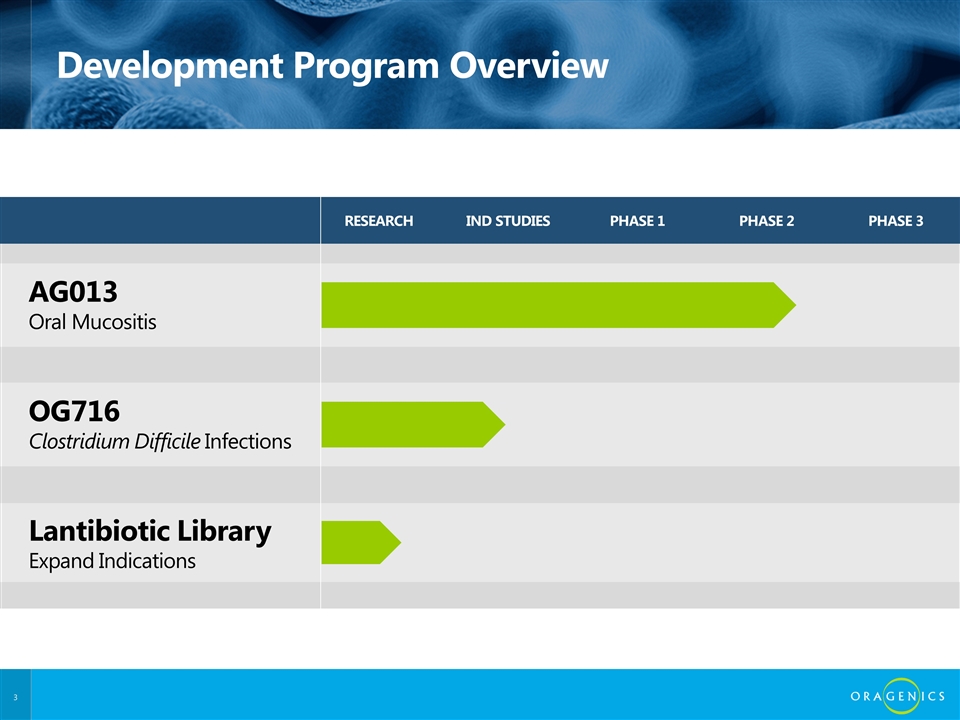
RESEARCH IND STUDIES PHASE 1 PHASE 2 PHASE 3 AG013 Oral Mucositis OG716 Clostridium Difficile Infections Lantibiotic Library Expand Indications Development Program Overview
Lantibiotics: Novel Platform of Antibiotics to Treat Serious Life-Threatening Infections Lantibiotics (aka lanthipeptide antibiotics) are naturally produced by variety of Gram-positive bacteria Prior development limited by manufacturing hurdles Platform: >700 lantibiotic structures created, potentially generating a pipeline of new compounds Platform provides potential for development in multidrug resistant infections: Virulent Clostridium difficile Methicillin Resistant Staphlococcus aureus (MRSA) Vancomycin Resistant Enterococci (VRE) Gram(-) infections Mutacin 1140: a lantibiotic produced by Streptococcus mutans
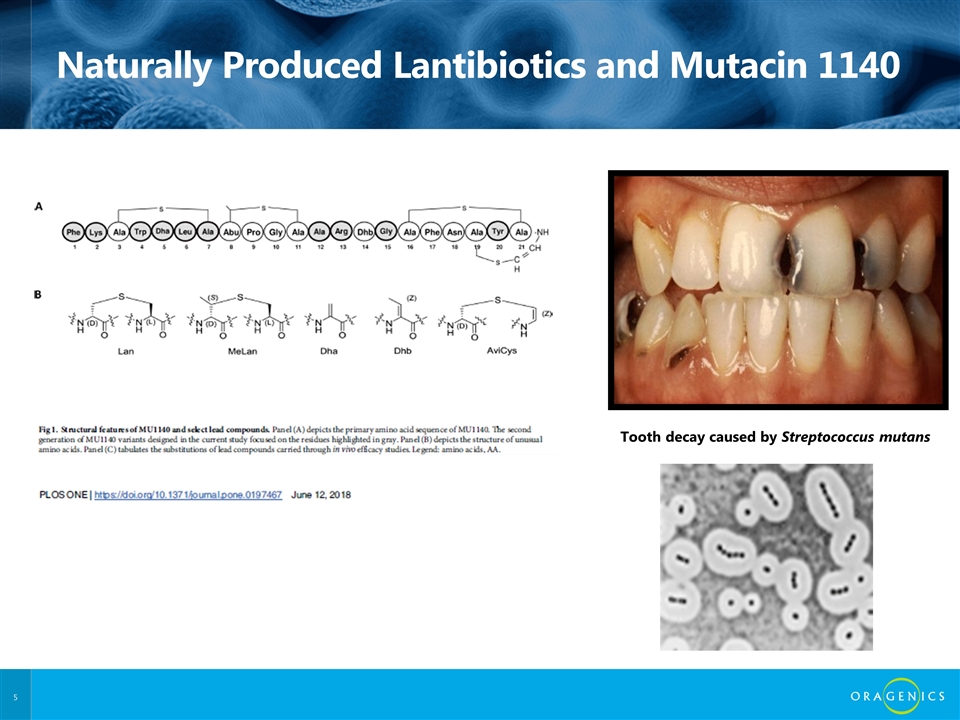
Naturally Produced Lantibiotics and Mutacin 1140 Tooth decay caused by Streptococcus mutans
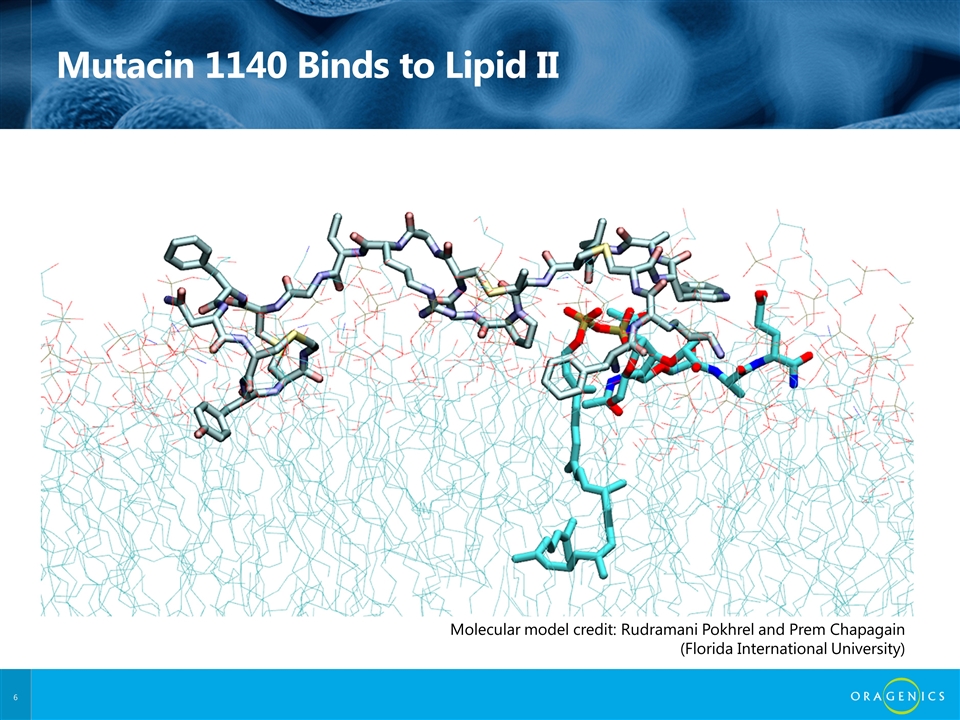
Mutacin 1140 Binds to Lipid II Molecular model credit: Rudramani Pokhrel and Prem Chapagain (Florida International University)

New MOA – Lipid II Abduction

Challenges in Developing a “Druggable” Lantibiotic Prior development limited by: 1-Manufacturability Low titers at fermentation Technical hurdles during solid-phase synthesis Ability to purify pharmaceutical grade compounds Process scalability 2- Physico-chemical and biological properties Sensitivity to proteolytic degradation
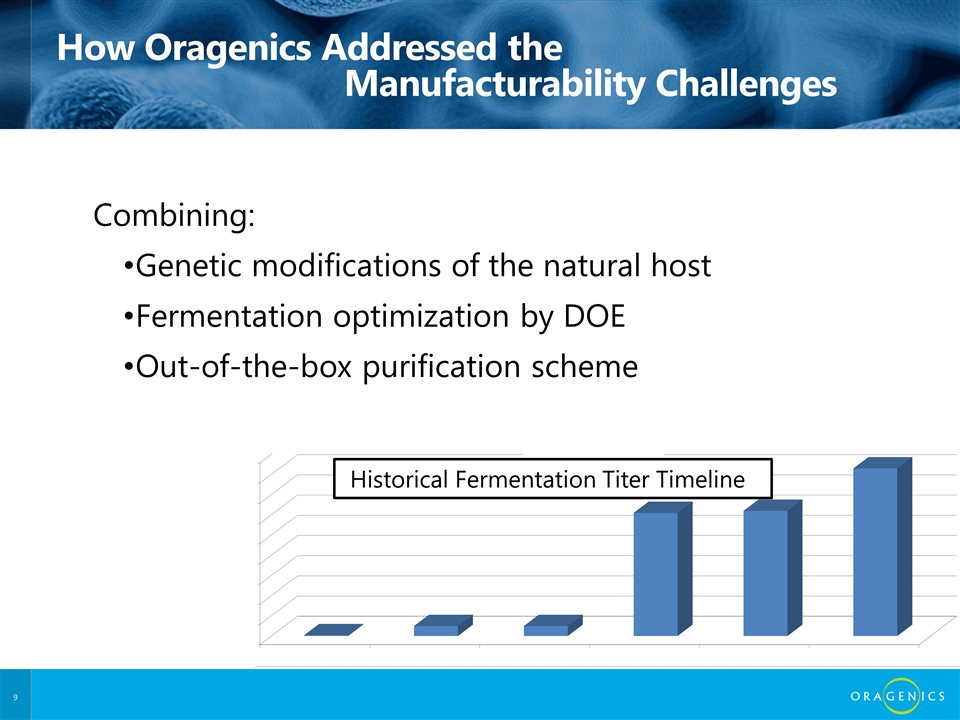
How Oragenics Addressed the Manufacturability Challenges Combining: Genetic modifications of the natural host Fermentation optimization by DOE Out-of-the-box purification scheme Historical Fermentation Titer Timeline
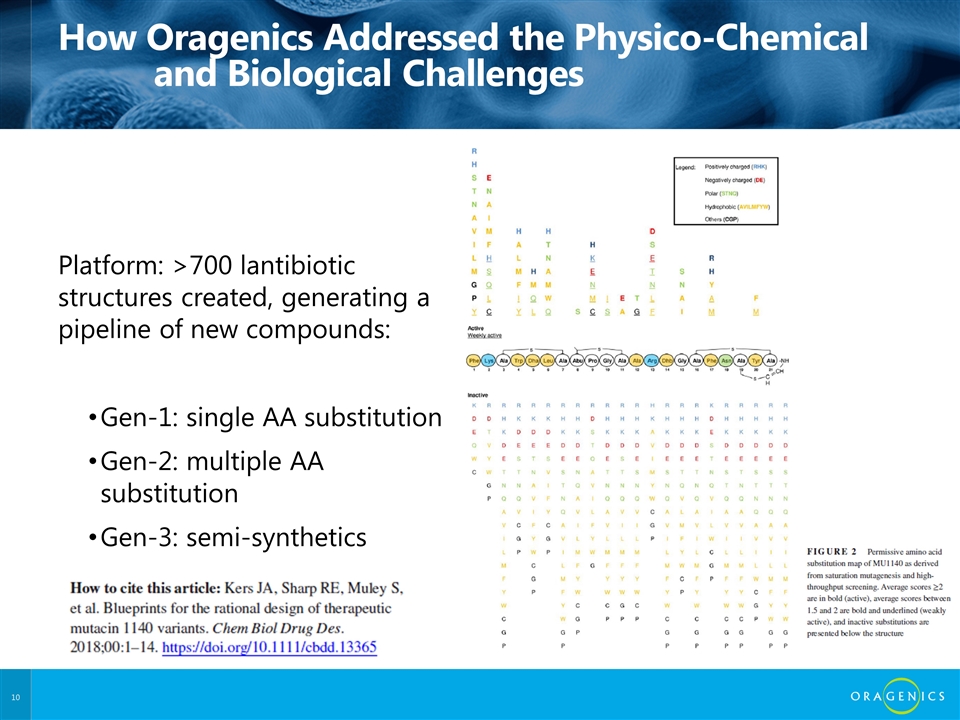
How Oragenics Addressed the Physico-Chemical and Biological Challenges Platform: >700 lantibiotic structures created, generating a pipeline of new compounds: Gen-1: single AA substitution Gen-2: multiple AA substitution Gen-3: semi-synthetics
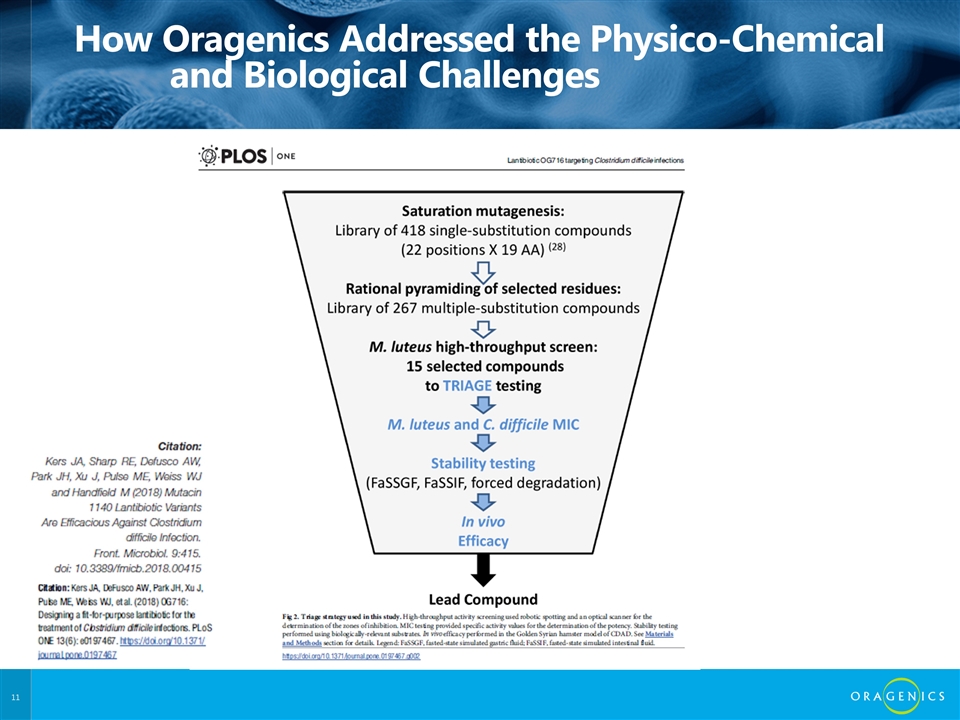
How Oragenics Addressed the Physico-Chemical and Biological Challenges
OG716 - Lantibiotic Lead against C. difficile Preliminary MU1140 (parent compound) preclinical data: Novel mechanism of action (unique binding to Lipid II) No cross-reactivity with existing classes of antibiotics Minimal in vitro cytotoxicity in mouse and human cell lines; minimal immunogenicity OG716 selected as lead compound for treatment of C. difficile infections Orally active Microbiology profile favorably compares to previous compounds Potent against Clostridium difficile in standard animal infection model Intellectual property extends into late 2030s for second-generation compounds
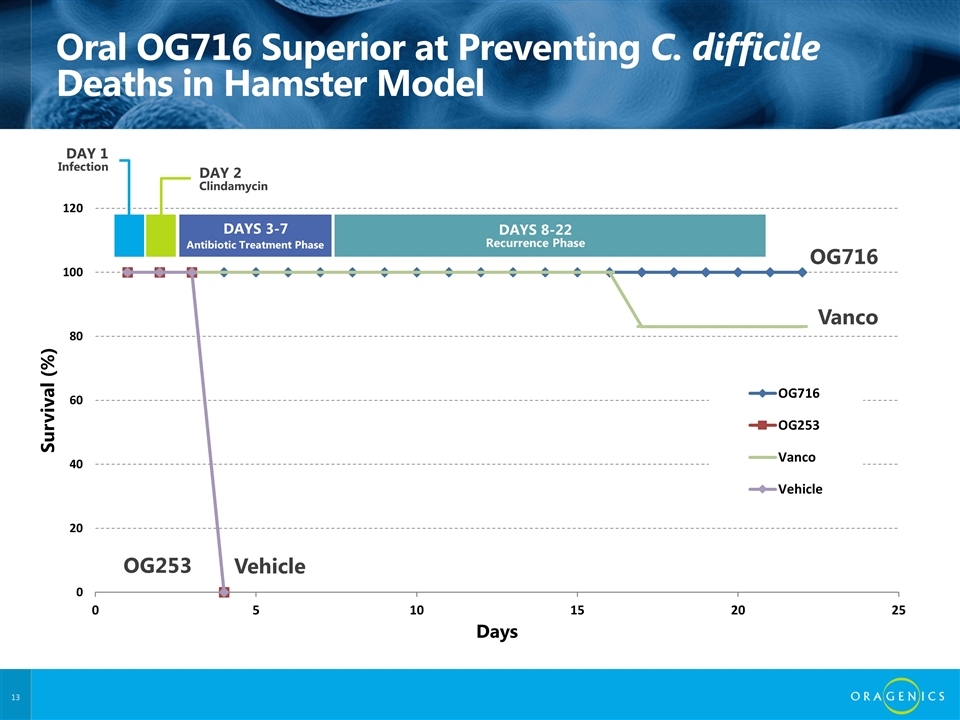
Oral OG716 Superior at Preventing C. difficile Deaths in Hamster Model OG716 Vanco Vehicle OG253 DAY 2 Clindamycin DAYS 3-7 DAYS 8-22 Recurrence Phase DAY 1 Infection Antibiotic Treatment Phase
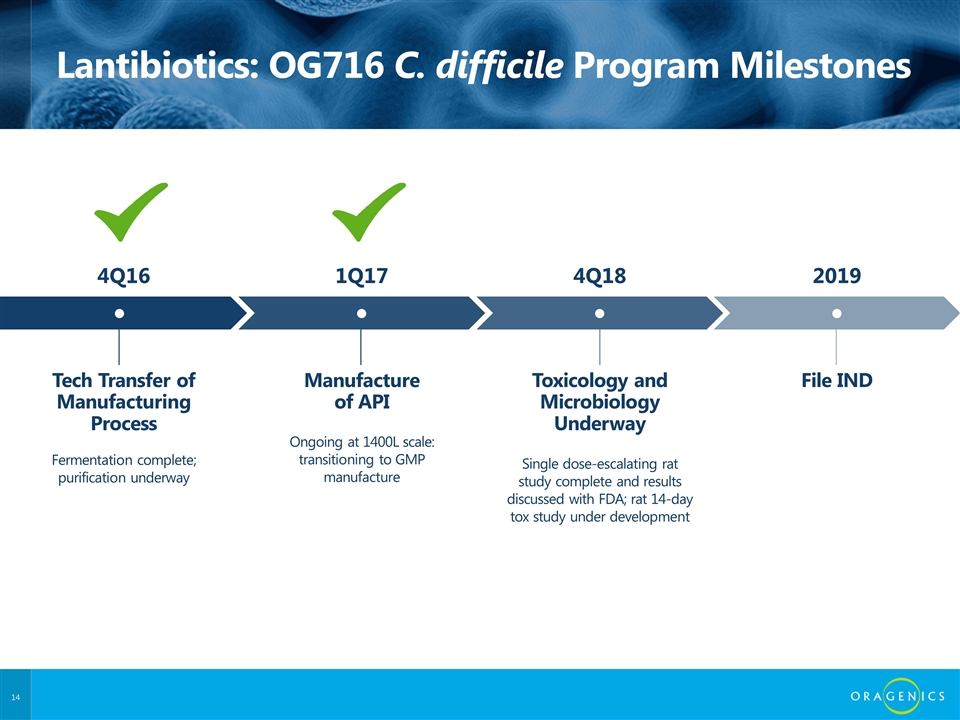
Lantibiotics: OG716 C. difficile Program Milestones Tech Transfer of Manufacturing Process ● Fermentation complete; purification underway 4Q16 Manufacture of API ● Ongoing at 1400L scale: transitioning to GMP manufacture 1Q17 Toxicology and Microbiology Underway ● Single dose-escalating rat study complete and results discussed with FDA; rat 14-day tox study under development 4Q18 File IND ● 2019

Achnowledgements Christopher McGee, Ph.D. , DeAnna Long, Ph.D., Lara Hurant, MBA, Matteo Villain, Ph.D., Andrea Lee and the rest of the Team Dr. Jeffrey D. Hillman Founder and CSO 1996-2010

APPENDIX
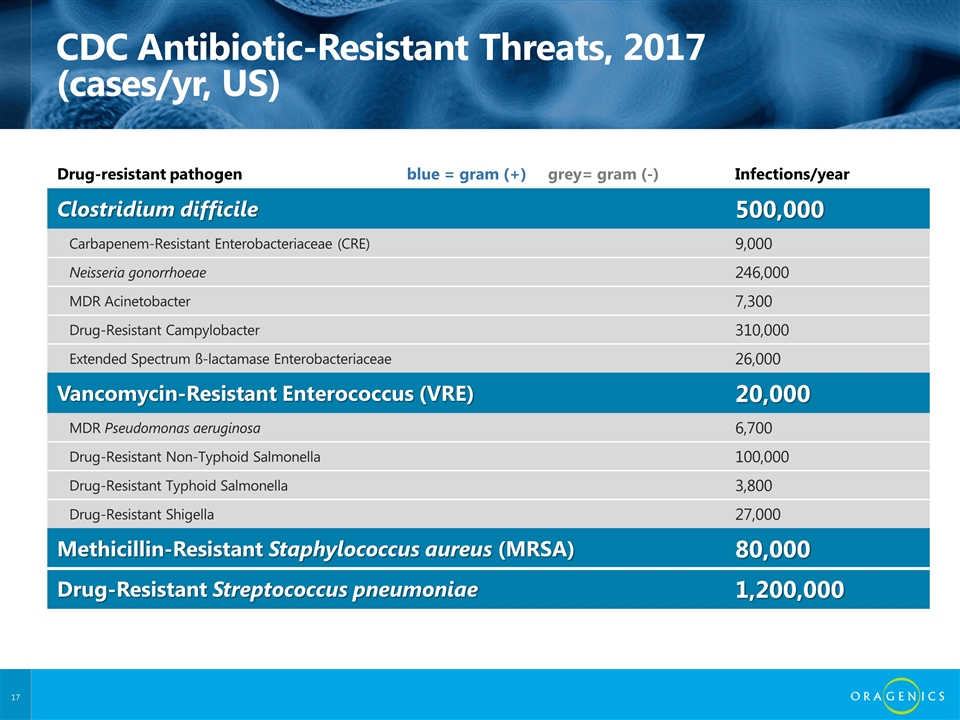
CDC Antibiotic-Resistant Threats, 2017 (cases/yr, US) Drug-resistant pathogen blue = gram (+) grey= gram (-) Infections/year Clostridium difficile 500,000 Carbapenem-Resistant Enterobacteriaceae (CRE) 9,000 Neisseria gonorrhoeae 246,000 MDR Acinetobacter 7,300 Drug-Resistant Campylobacter 310,000 Extended Spectrum ß-lactamase Enterobacteriaceae 26,000 Vancomycin-Resistant Enterococcus (VRE) 20,000 MDR Pseudomonas aeruginosa 6,700 Drug-Resistant Non-Typhoid Salmonella 100,000 Drug-Resistant Typhoid Salmonella 3,800 Drug-Resistant Shigella 27,000 Methicillin-Resistant Staphylococcus aureus (MRSA) 80,000 Drug-Resistant Streptococcus pneumoniae 1,200,000
C. difficile and C. difficile Infection (CDI): Epidemiology C. difficile is an infection of the colon causing colitis by producing toxins that damage lining of the colon 500,000 infections annually resulting in 29,000 deaths 83,000 will experience at least one recurrence Deaths have increased 400% since 2000 Healthcare-associated infections occur: 37% hospital onset, 36% nursing home onset, 27% community onset C. difficile associated diarrhea is associated with a 1-2 week hospital stay Emerging problem: 8% of CDI associated with onset of concomitant Vancomycin Resistant Enterococci (VRE) infection

Competitive Overview Currently Approved Therapies: Metronidazole Vancomycin Fidaxomicin Rifaximin Zinplava (monoclonal antibody) Therapies under development: Follow-on generations of existing antibiotics, enzymes and enzyme/protein synthesis inhibitors, vaccines, microbiome/fecal transplant therapies, and toxin binding polyclonal antibodies. * Source: GlobalData Projected 2019 U.S. sales for C. difficile: $426M*