
Filed Pursuant to Rule 424(b)(5)
Registration No. 333-269225
This preliminary prospectus supplement relates to an effective registration statement under the Securities Act of 1933, as amended, but the information in this preliminary prospectus supplement is not complete and may be changed. This preliminary prospectus supplement and the accompanying prospectus are not an offer to sell these securities, and we are not soliciting offers to buy these securities in any state or other jurisdiction where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS SUPPLEMENT | SUBJECT TO COMPLETION | DATED JANUARY 16, 2024 |
(To Prospectus dated January 25, 2023)
Shares of Common Stock
Pre-Funded Warrants to Purchase Common Stock

Oragenics, Inc.
We are offering shares of our common stock, $0.001 par value per share (the “common stock”), at an offering price of $ per share, pursuant to this prospectus supplement and the accompanying base prospectus.
We are also offering pre-funded warrants (each a “Pre-funded Warrant”) to purchase shares of our common stock, exercisable at an exercise price of $0.001 per share, to those purchasers whose purchase of common stock in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering. The purchase price of each Pre-funded Warrant is equal to the price per share of common stock being sold to the public in this offering, minus $0.001. The Pre-funded Warrants will be immediately exercisable and may be exercised at any time until all of the Pre-funded Warrants are exercised in full.
Our common stock is listed on the NYSE American under the symbol “OGEN”. The last reported sale price of our common stock on the NYSE American on January 12, 2024 was $6.06 per share. There is no established trading market for the Pre-funded Warrants and we do not intend to list the Pre-funded Warrants on any securities exchange or nationally recognized trading system.
As of January 3, 2024, the aggregate market value of our outstanding common stock held by non-affiliates, or the public float, was approximately $4,701,675, which was calculated based on 2,062,138 shares of our outstanding common stock held by non-affiliates and on a price of $6.84 per share, which is the highest closing price of our common stock on the NYSE American within the prior 60 days. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell our securities in a public primary offering with a value exceeding one-third of our public float in any 12-month period unless our public float subsequently rises to $75.0 million or more. During the 12-calendar month period that ends on, and includes, the date of this prospectus supplement (but excluding this offering), we have not offered and sold any of our securities pursuant to General Instruction I.B.6 of Form S-3.
Investing in our securities involves significant risks. Please read the information contained in or incorporated by reference under the heading “Risk Factors” beginning on page S-16 of this prospectus supplement, and under similar headings in other documents filed after the date hereof and incorporated by reference into this prospectus supplement and the accompanying prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Share | Per Pre-funded Warrant | Total | ||||||||||
| Public offering price | $ | $ | $ | |||||||||
| Underwriting discount(1) | $ | $ | $ | |||||||||
| Proceeds, before expenses, to us | $ | $ | $ | |||||||||
| (1) | See “Underwriting” beginning on page S-28 for additional information regarding underwriting compensation. |
We have granted a 45-day option to the representative of the underwriters to purchase up to additional shares of common stock and/or additional Pre-funded Warrants to purchase up to additional shares of common stock solely to cover over-allotments, if any.
The underwriter expects to deliver the securities to purchasers on or about , 2024.
ThinkEquity
The date of this prospectus supplement is , 2024
TABLE OF CONTENTS
Prospectus Supplement
| Page | |
| ABOUT THIS PROSPECTUS SUPPLEMENT | S-i |
| CAUTIONARY STATEMENT CONCERNING FORWARD-LOOKING STATEMENTS | S-ii |
| PROSPECTUS SUPPLEMENT SUMMARY | S-1 |
| THE OFFERING | S-1 |
| RISK FACTORS | S-16 |
| USE OF PROCEEDS | S-25 |
| DIVIDEND POLICY | S-25 |
| DESCRIPTION OF PRE-FUNDED WARRANTS | S-25 |
| DILUTION | S-27 |
| UNDERWRITING | S-28 |
| LEGAL MATTERS | S-31 |
| EXPERTS | S-31 |
| WHERE YOU CAN FIND MORE INFORMATION | S-31 |
| INFORMATION INCORPORATED BY REFERENCE | S-32 |
Prospectus
ABOUT THIS PROSPECTUS SUPPLEMENT
This document is part of the registration statement on Form S-3 that we filed with the Securities and Exchange Commission, or the SEC, using a “shelf” registration process (Registration File No. 333-265995) and consists of two parts. The first part is this prospectus supplement, which describes the specific terms of this offering. The second part, the accompanying prospectus, gives more general information, some of which may not apply to this offering. Generally, when we refer only to the “prospectus,” we are referring to both parts combined. This prospectus supplement may add to, update or change information in the accompanying prospectus and the documents incorporated by reference into this prospectus supplement or the accompanying prospectus.
If information in this prospectus supplement is inconsistent with the accompanying prospectus or with any document incorporated by reference that was filed with the SEC before the date of this prospectus supplement, you should rely on this prospectus supplement. This prospectus supplement, the accompanying prospectus and the documents incorporated into each by reference include important information about us, the securities being offered and other information you should know before investing in our securities. You should also read and consider information in the documents we have referred you to in the sections of this prospectus supplement entitled “Where You Can Find More Information” and “Incorporation of Certain Information by Reference”.
You should rely only on this prospectus supplement, the accompanying prospectus, the documents incorporated or deemed to be incorporated by reference herein or therein and any free writing prospectus prepared by us or on our behalf. We have not authorized anyone to provide you with information that is in addition to or different from that contained or incorporated by reference in this prospectus supplement and the accompanying prospectus. If anyone provides you with different or inconsistent information, you should not rely on it. We are not offering to sell these securities in any jurisdiction where the offer or sale is not permitted. You should not assume that the information contained in this prospectus supplement, the accompanying prospectus or any free writing prospectus, or incorporated by reference herein, is accurate as of any date other than as of the date of this prospectus supplement or the accompanying prospectus or any free writing prospectus, as the case may be, or in the case of the documents incorporated by reference, the date of such documents regardless of the time of delivery of this prospectus supplement and the accompanying prospectus or any sale of our securities. Our business, financial condition, liquidity, results of operations and prospects may have changed since those dates.
References to, “we,” “us,” “our company,” “Oragenics,” the “Company,” and similar terms refer to Oragenics, Inc., a Florida corporation, unless the context otherwise requires.
No action is being taken in any jurisdiction outside the United States to permit a public offering of the securities or possession or distribution of this prospectus supplement or the accompanying prospectus in that jurisdiction. Persons who come into possession of this prospectus supplement or the accompanying prospectus in jurisdictions outside the United States are required to inform themselves about, and to observe, any restrictions as to this offering and the distribution of this prospectus supplement or the accompanying prospectus applicable to that jurisdiction.
The industry and market data and other statistical information contained in this prospectus supplement, the accompanying prospectus and the documents we incorporate by reference are based on management’s estimates, independent publications, government publications, reports by market research firms or other published independent sources, and, in each case, are believed by management to be reasonable estimates. Although we believe these sources are reliable, we have not independently verified the information. None of the independent industry publications used in this prospectus supplement, the accompanying prospectus or the documents we incorporate by reference were prepared on our or our affiliates’ behalf and none of the sources cited by us consented to the inclusion of any data from its reports, nor have we sought their consent.
We further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference herein were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
| S-i |
CAUTIONARY STATEMENT CONCERNING FORWARD-LOOKING STATEMENTS
Certain statements in this prospectus supplement, the accompanying prospectus and documents incorporated by reference herein that look forward in time or express management’s expectations or beliefs with respect to the occurrence of future events are forward-looking statements as defined under within the meaning of Section 27A of the Securities Act of 1933, as amended, or Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or Exchange Act, as amended, and are subject to the safe harbor created therein for forward-looking statements. Such statements include, but are not limited to, (i) projections of revenue, earnings, capital structure and other financial items, (ii) statements of our plans and objectives, (iii) statements of expected future economic performance, and (iv) assumptions underlying statements regarding us or our business. Forward-looking statements can be identified by, among other things, the use of forward-looking language, such as “believes,” “expects,” “estimates,” “may,” “will,” “should,” “could,” “seeks,” “plans,” “intends,” “anticipates” or “scheduled to” or the negatives of those terms, or other variations of those terms or comparable language, including discussions of strategy or other intentions, particularly as they relate to the development and funding of our product candidates.
We caution investors that actual results or business conditions may differ materially from those projected or suggested in forward-looking statements as a result of various factors including, but not limited to, the following risks and the other factors described in the Risk Factors section of this prospectus supplement, our annual report on Form 10-K, in our quarterly reports on Form 10-Q and in our Current Reports on Form 8-K incorporated by reference. These factors include:
| ● | Our significant operating losses, which we have incurred since our inception, and our inability to assure you that we will generate revenues or achieve profitability in the future; | |
| ● | Our need for, and ability to raise, additional capital; | |
| ● | Our expectations related to the use of proceeds from this Offering; | |
| ● | The timing, progress and results of clinical trials of our product candidates, including statements regarding the timing of initiation and completion of pre-clinical studies or clinical trials or related preparatory work, the period during which the results of the trials will become available and our research and development programs; | |
| ● | Our ability to timely and successfully achieve the anticipated benefits of acquiring the assets that we recently acquired from Odyssey Health, Inc., including ONP-002, which is a unique neurosteroid drug compound intended to treat mild traumatic brain injuries also known as concussions; | |
| ● | Our expectations regarding the potential benefits, activity, effectiveness and safety of our product candidates including as to administration, distribution and storage; | |
| ● | Our expectations regarding the size of the patient populations, market acceptance and opportunity for and clinical utility of our product candidates, if approved for commercial use; | |
| ● | Our manufacturing capabilities and strategy, including the scalability and commercial viability of our manufacturing methods and processes, and those of our contractual partners; | |
| ● | Our expectations regarding the scope of any approved indications for our product candidates; | |
| ● | Our ability to successfully commercialize our product candidates; | |
| ● | The potential benefits of, and our ability to maintain, our relationships and collaborations with the NIAID, the NIH, the NRC and other potential collaboration or strategic relationships;
| |
|
● | Our estimates of our expenses, ongoing losses, future revenue, capital requirements and our needs for or ability to obtain additional funding, including any application for future grants or funding; |
| ● | Our ability to identify, recruit and retain key personnel and consultants; |
| S-ii |
| ● | Our ability to obtain, retain, protect and enforce our intellectual property position for our product candidates, and the scope of such protection; | |
| ● | Our ability to advance the development of our product candidates under the timelines and in accord with the milestones projected; | |
| ● | Our need to comply with extensive and costly regulation by worldwide health authorities, who must approve our product candidates prior to substantial research and development and could restrict or delay the future commercialization of certain of our product candidates; | |
| ● | Our ability to successfully complete pre-clinical and clinical development of, and obtain regulatory approval of our product candidates and commercialize any approved products on our expected timeframes or at all; | |
| ● | The content and timing of submissions to and decisions made by the FDA, other regulatory agencies and nongovernmental bodies and actors, such as investigational review boards; | |
| ● | The effects of government regulation and regulatory developments, and our ability and the ability of the third parties with whom we engage to comply with applicable regulatory requirements; | |
| ● | The capacities and performance of our suppliers and manufacturers and other third parties over whom we have limited control; | |
| ● | Our ability to maintain our listing on the NYSE American and the effects of our recently completed acquisition of assets from Odyssey Health, Inc.; | |
| ● | The impact of the COVID-19 pandemic on our financial condition and business operations and our ability to continue research and development for existing product candidates on previously-projected timelines or in accord with ordinary practices, as well as the broader governmental, global health and macro- and microeconomic responses to and consequences of the pandemic; | |
| ● | We may be adversely impacted by any significant broad-based financial crises and its impact on consumers, retailers and equity and debt markets as well as our inability to obtain required additional funding to conduct our business; | |
| ● | As a public company, we must implement additional and expensive finance and accounting systems, procedures and controls as we grow our business and organization to satisfy reporting requirements, which add to our costs and require additional management time and resources; | |
| ● | Our competitive position and the development of and projections relating to our competitors or our industry; and | |
| ● | The impact of laws and regulations, including those that may not yet exist. |
We cannot assure you that we have identified all the factors that create uncertainties. Moreover, new risks emerge from time to time and it is not possible for our management to predict all risks, nor can we assess the impact of all risks on our business or the extent to which any risk, or combination of risks, may cause actual results to differ from those contained in any forward-looking statements.
We urge you to consider these factors before investing in our common stock. The forward-looking statements included in this prospectus supplement, the accompanying prospectus and any other offering material, or in the documents incorporated by reference into this prospectus supplement, the accompanying prospectus and any other offering material, are made only as of the date of the prospectus supplement, the accompanying prospectus, any other offering material or the incorporated document. Except as required by law, we undertake no obligation to publicly release the result of any revision of these forward-looking statements to reflect events or circumstances after the date of this prospectus or the respective dates of documents incorporated by reference herein or therein that include forward-looking statements.
This prospectus supplement also contains estimates, projections and other information concerning our industry, the market and our business. Information that is based on estimates, forecasts, projections or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. We obtained the industry, market and competitive position data in this prospectus from our own internal estimates and research as well as from industry and general publications and research surveys and studies conducted by third parties.
You should consider all risks and uncertainties disclosed in our filings with the SEC described in the sections of this prospectus supplement entitled “Risk Factors,” “Where You Can Find Additional Information” and “Incorporation of Certain Information by Reference,” all of which are accessible on the SEC’s website at www.sec.gov.
| S-iii |
This summary highlights selected information contained elsewhere or incorporated by reference in this prospectus supplement and the accompanying prospectus. This summary may not contain all of the information that may be important to you. You should read this prospectus supplement, the accompanying prospectus, the information incorporated by reference in each, and any related free writing prospectus before making an investment decision. You should pay special attention to the “Risk Factors” section beginning on page S-16 of this prospectus supplement and “Risk Factors” set forth in our most recent annual report on Form 10-K for the year ended December 31, 2022, our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2023, June 30, 2023, and September 30, 2023, respectively and in the other documents which are incorporated by reference in this prospectus supplement and the accompanying prospectus in their entirety to determine whether an investment in our common stock is appropriate for you.
| Common stock we are offering | shares of common stock | |
| Pre-funded Warrants we are offering | We are also offering Pre-funded Warrants to purchase up to shares of common stock, to those purchasers whose purchase of common stock in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering. Each Pre-Funded Warrant entitles the holder to purchase one share of common stock at an exercise price of $0.001 per share. The purchase price of each Pre-Funded Warrant is equal to the price per share of common stock being sold to the public in this offering minus $0.001. The Pre-Funded Warrants will be immediately exercisable and may be exercised at any time until exercised in full. | |
| Underwriter’s over-allotment option | We have granted a 45-day option to the underwriters to purchase up to an additional shares of common stock and/or up to an additional Pre-Funded Warrants, representing 15% of the shares of common stock and/or Pre-Funded Warrants sold in the offering, in each case, solely to cover over-allotments, if any. | |
| Common stock outstanding prior to this offering | 3,080,693 shares of common stock. | |
| Common stock to be outstanding after this offering | shares of common stock, assuming the full exercise of the Pre-funded Warrants. | |
| NYSE American Symbol and Listing | Our common stock is listed on the NYSE American under the symbol “OGEN”. There is no established trading market for the Pre-funded Warrants and we do not intend to list the Pre-funded Warrants on any securities exchange or nationally recognized trading system. | |
| Use of Proceeds | We estimate that the net proceeds from this offering, after payment of estimated offering expenses payable by us and underwriting discounts will be approximately $ million. We intend to use the net proceeds from this offering to fund the continued development of ONP-002, which is a unique neurosteroid drug compound intended to treat mild traumatic brain injuries also known as concussions, and for general corporate purposes and working capital. See “Use of Proceeds”. |
| S-1 |
| Lock-up Agreements | Our executive officers and directors have agreed with the underwriters not to sell, transfer or dispose of any shares or similar securities for a period of three months from the date of this prospectus supplement. For additional information regarding our arrangement with the underwriters, please see “Underwriting”. | |
| Risk Factors | Investing in our securities involves significant risks. Please read the information contained in or incorporated by reference under the heading “Risk Factors” beginning on page S-16 of this prospectus supplement, and under similar headings in other documents filed after the date hereof and incorporated by reference into this prospectus supplement and the accompanying prospectus. |
The number of shares of common stock shown above to be outstanding after this offering is based on 3,080,693 shares outstanding as of January 16, 2024 and excludes:
| ● | 271,194 shares of our common stock issuable upon the exercise of outstanding options under our equity incentive plans at a weighted average exercise price of $18.83 per share; | |
| ● | 260,995 shares of common stock reserved for issuance under outstanding warrants with a weighted average exercise price of $79.04 per share; | |
| ● | 1,004,235 additional shares of common stock reserved for future issuance under our 2021 equity incentive plan; | |
| ● | approximately 9,028 shares of common stock reserved for issuance under conversion of our outstanding shares of Series A Non-Voting, Convertible Preferred Stock; | |
| ● | approximately 13,500 shares of common stock reserved for issuance under conversion of our outstanding shares of Series B Non-Voting, Convertible Preferred Stock; and | |
| ● | approximately 7,488,692 shares of common stock reserved for issuance under conversion of 7,488,692 outstanding shares of Series F Non-Voting, Convertible Preferred Stock. |
Except as otherwise indicated, all information in this prospectus supplement assumes no exercise by the underwriter of its option to purchase additional shares.
| S-2 |
Overview
We are a development-stage biotechnology company focused on nasal delivery of pharmaceutical medications in neurology and fighting infectious diseases. On December 28, 2023, we successfully consummated our previously announced Asset Purchase Agreement with Odyssey Health, Inc. (“Odyssey”), pursuant to which we purchased all of Odyssey’s assets related to the segment of Odyssey’s business focused on developing medical products that treat brain related illnesses and diseases (the “Neurology Assets”).
The Neurology Assets include drug candidates for treating mild traumatic brain injury (mTBI), also known as concussion, and for treating Niemann Pick Disease Type C (NPC), as well as Odyssey’s proprietary powder formulation and its intranasal delivery device.
As a result of the acquisition of the Neurology Assets, we expect that, in the near- and mid-terms, we will focus our resources and efforts on the continued development of the Neurology Assets and primarily ONP-002, which, as discussed further below, has successfully completed phase 1 clinical trials. The acquisition is expected to build on our expertise in intranasal platforms and expand our portfolio into more areas of unmet medical needs. Nasal delivery offers many advantages over standard systemic delivery systems, such as its non-invasive character, a fast onset of action and in many cases reduced side effects due to a more targeted delivery.
We will concurrently determine how best to proceed with the development of our nasal COVID-19 product candidate, given our limited resources, and for the time being, we anticipate placing our lantibiotics program on hold.
In conjunction with the Neurology Asset acquisition, we paid Odyssey a total of $1,000,000 in cash, $500,000 of which was paid in October, 2023 and $500,000 of which was paid on December 11, 2023. In addition, at the closing, we issued Odyssey 8,000,000 shares of our newly created Series F Non-Voting Convertible Preferred Stock, which are convertible into our common stock on a one-to-one basis (subject to certain adjustments). Odyssey converted 511,308 of those shares into our common stock on December 28, 2023. Our Certificate of Designation creating the Series F Preferred Stock specifies that the remainder of the shares are not convertible until the occurrence of all of the following: (i) Oragenic’s shall have applied for and been approved for initial listing on the NYSE American or another national securities exchange or shall have been delisted from the NYSE American, which Oragenic’s does not anticipate undertaking until it meets the NYSE American’s initial listing standards, and (ii) if required by the rules of the NYSE American, Oragenic’s shareholders shall have approved any change of control that could be deemed to occur upon the conversion of the Series F Preferred Stock into common stock, based on the fact and circumstances existing at such time.
Upon the closing of the Neurology Asset acquisition, Michael Redmond, who has served as President and CEO of Odyssey since 2018, was named President of Oragenics. Mr. Redmond has 35 years of commercial experience with medical device companies, having held various sales and marketing leadership positions that helped accelerate growth at companies to multiples of their previous revenue and valuation. Mr. Redmond also has significant experience in raising capital and securing licensing and distribution deals with major biotech and pharmaceutical companies. In his new position, Mr. Redmond will oversee the growth of Oragenics’ neurology product pipeline and intranasal drug delivery technologies. Additionally, the Odyssey management and development team that led the ONP-002 clinical trial design and implementation for the treatment of concussion, will continue to oversee research and development of the newly acquired Neurology Assets at Oragenics. The team has experience in conducting clinical trials, developing drug formulations and commercializing pharmaceutical products across a broad range of indications.
About Mild Traumatic Brain Injury (mTBI)
Concussions are an unmet medical need that affects millions worldwide. Repetitive concussions can increase the risk of developing chronic traumatic encephalopathy and other neuropsychiatric disorders. It is estimated there is upwards of 3.8M million sports-related concussions alone in the U.S. annually and that up to 50% go unreported (Hallock et al., 2023). The worldwide incidence is estimated at 69 million per year (Dewan et al., 2018). The global market for concussion treatment was valued at $6.9 billion in 2020 and is forecast to reach $8.9 billion by 2027, according to Grandview Research. Common settings for concussion include contact sports, military training and operations, motor vehicle accidents, children at play and elderly assistive-living facilities due to falls.
| S-3 |
About ONP-002
The Neurology Assets acquired from Odyssey include ONP-002 and a unique nasal delivery device, Odyssey’s lead concussion assets, believed to be a first-in-class intranasal drug under development for the treatment of moderate-to-severe concussion in the acute through subacute phases (mTBI (concussion)).
ONP-002 is a fully synthetic, non-naturally occurring neurosteroid, is lipophilic, and can cross the blood-brain barrier to rapidly eliminate swelling, oxidative stress and inflammation while restoring proper blood flow through gene amplification.
ONP-002 to date has been shown to be stable up to 104 degrees for 18-months. The drug candidate is spray-dry manufactured into a powder and filled into the novel intranasal device. The drug is then administered through the nasal passage from the device. The novel intranasal device is lightweight and easy to use in the field.
The proprietary powder formulation and intranasal administration allows for rapid and direct accessibility to the brain. The device is breath propelled and Oragenics expects it to allow patients to blow into the device which closes the soft palate in the back of the nasopharynx, preventing the flow of drug to the lungs or esophagus, minimizes system exposure and side effects, and easily crosses the blood brain barrier. This mechanism traps ONP-002 in the nasal cavity allowing for more abundant and faster drug availability in the traumatized brain.
Expected ONP-002 Product Development Timeline:
| Pre-clinical Animal Studies | Phase 1 | Phase 2a | Phase 2b | Phase 3 | ||||
| Complete | Complete | Estimated May 2024 start | Estimated November 2024 start | Estimated November 2026 start |
This product development plan is an estimate and is subject to change based on funding, technical risks and regulatory approvals.
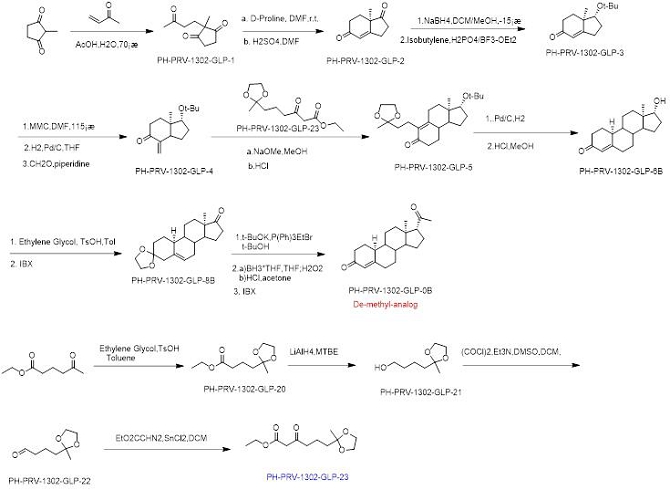
ONP-002 Structure and Synthesis. Pharmaron, Inc. is our current partner in the development of synthetic chemistry and manufacturing of the ONP-002 (Molecular Formula: C20H28O2, Molecular Weight: 300.14 g/mol). ONP-002 is the enantiomer (mirror image) of 19-Norprogesterone, a novel chemical entity based on its chiral purity and optical rotation. There are two components to this synthesis. The synthesis of PH-PRV-1302-GLP-23 (in blue) and the synthesis making use of PH-PRV-1302-GLP-23 to append the side chain necessary in ONP-002 production. ONP-002 is red in Figure 1 and defined as the De-methyl- analog. The current API was made under GMP standards.
| S-4 |
Figure 1
Validation and Stability of ONP-002. Pharmaron issued a Certificate of Analysis (CoA, Appendix A-1). Testing methods were standard and include appearance, identification by 1H NMR, identification by Mass Spectroscopy (MS), optical purity by HPLC, residual solvent analysis, elemental impurities, percent water, and residue on ignition. Pharmaron has shown both the specifications and the results, indicating that the material supplied passes all criteria. ONP-002 is supplied by Pharmaron in pure form. As such, no excipients are present. Stability studies were performed by storing samples under carefully controlled conditions with respect to temperature and humidity. Pharmaron’s stability testing protocol included storage at 25 °C± 2 °C at 60% relative humidity ± 5% relative humidity for 24 months and 40 °C± 2 °C at 75% relative humidity ± 5% for 18 months (Appendix A-2). Samples are pulled at the scheduled time and analyzed for appearance, purity, assay, optical purity, and water content. No changes in ONP-002 were seen.
Formulation. The ONP-002 drug product formulation contains the active drug substance, ent-19-Norprogesterone with hydroxypropyl beta cyclodextrin (HPβCD) creating nanoparticles. Formulation of the product is achieved through solubilization of ONP-002 with HPβCD followed by spray drying and packaging into a breath-propelled intranasal delivery device. The spray-dried powder provides for a particle size between 11 and 12 microns upon dispersion from the device. For placebo dosing, the breath-propelled intranasal delivery device is pre-packaged with HPβCD only with no ONP-002 active drug substance. The highest achieved concentration of ONP-002 per dose is currently at 8% or 8mg/100mg given each intranasal dose is designed to deliver 100mg of the spray-dried formula. Studies have been designed to improve the final % of API as high as 32%.
Intellectual Property. Patents on ONP-002 have been filed and/or issued and a patent has been filed on the nasal delivery device as follows:
| ● | New chemical entity IP filing– USPTO pending, approved Europe and Canada |
| ○ | C-20 steroid compounds, composition and uses thereof to treat traumatic brain injury (TBI), including concussion. |
| ○ | The invention relates to ONP-002 composition and methods of use thereof to treat, minimize and/or prevent traumatic brain injury (TBI), including severe TBI, moderate TBI, and mild TBI, including concussions. |
| ○ | Patent expiration with max patent term extension – 9/17/2040 |
| ○ | Patent expiration with no patent term extension – 9/17/2035 |
| ● | Method of intranasal delivery and device components – USPTO pending |
ONP-002 Pre-Clinical Trials
The drug has completed toxicology studies in rats and dogs. Studies show that ONP-002 has a safety margin over 90X its predicted efficacious dose. In preclinical animal studies, the asset demonstrated rapid and broad biodistribution throughout the brain while simultaneously reducing swelling, inflammation and oxidative stress, along with an excellent safety profile.
Results from the preclinical studies suggest that ONP-002 has an equivalent, and potentially superior, neuroprotective effect compared to related neurosteroids. The animals treated with the drug post-concussion showed positive behavioral outcomes using various testing platforms including improved memory and sensory-motor performance, and reduced depression/anxiety like behavior.
Below is a detailed analysis of our pre-clinical data.
ONP-002 Induction of PXR. The induction of the human CYP450 enzymes, CYP2B6, and CYP3A4 by ONP-002, as measured by mRNA expression, was tested in human hepatocytes from 3 donors at 3 concentrations: 1 μM, 10 μM and 100 μM. (Table 1). We show that ONP-002 through the known PXR-mechanism produced a modest induction of CYP3A4, up to 17% of the positive control, and a greater induction of CYP2B6, of up to 59% of the positive control, both at a concentration of 100 μM. Past data shows that ONP-001 (ent-Progesterone) and Progesterone induce the PXR receptor (1). Receptor binding studies have been performed showing neither ONP-001 or -002 activate the classical Progesterone Receptor.
| S-5 |
Table 1
| Compound | Concentration μM | CYP2B6 Fold induction | % Of positive control | CYP3A4 Fold induction | % Of positive control | |||||||||
| Mean | SD | Mean | Mean | SD | Mean | |||||||||
| Rifampicin | 30 | - | - | - | 18 | 9.9 | 100 | |||||||
| Phenobarbital | 1000 | 5.1 | 2.9 | 100 | - | - | - | |||||||
| Omeprazole | 50 | - | - | - | - | - | - | |||||||
| Flumazenil (neg control) | 30 | 1.1 | 0.1 | 21 | 1.1 | 0.3 | 6 | |||||||
| ONP-002 | 1 | 1 | 0.2 | 19 | 1.3 | 0.3 | 7 | |||||||
| 10 | 1.6 | 0.6 | 31 | 2.4 | 0.6 | 13 | ||||||||
| 100 | 3.0 | 2.6 | 59 | 3.0 | 2.1 | 17 | ||||||||
ONP-002 Animal Studies. All surgical animals (male Sprague-Dawley rats approx. 250 grams) were anesthetized with an initial isoflurane induction for 4 min-the minimum time necessary to sedate the animal. The scalp was shaved and cleaned with isopropanol and betadine. During the stereotaxic surgery, anesthesia was maintained with isoflurane. A medial incision was made, and the scalp was pulled back over the medial frontal cortex. A 6-mm diameter craniotomy was performed exposing the brain tissue. An electrically controlled injury device using a 5 mm metal impactor was positioned over the exposed brain. An impact speed of 1.6 m/s at a 90-degree angle from vertical was used to produce an open head injury at a depth of 1mm to create a milder TBI. All treatments were given intranasal (IN) as a liquid solution with a micro atomizer. Vehicle for all administrations was 22.5% Hydroxy-Propyl-β-cyclodextrin (HPβCD).
Molecular Studies - Brain tissue was taken from the penumbral region of injury.
Figure 2
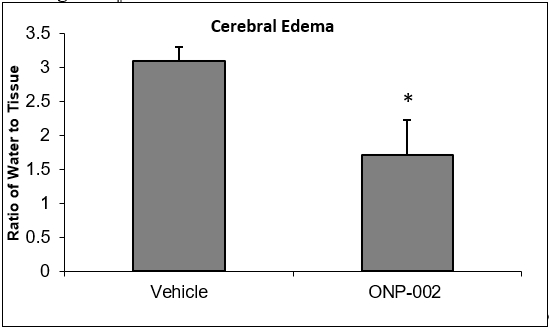
Cerebral Edema. In Figure 2, we show that ONP-002 reduces swelling in rats compared to vehicle-treated at 24-hrs after brain injury by measure of brain water content through speed-vacuum dehydration and tissue weight comparisons. ONP-002-treated (4mg/kg) and vehicle-treated were compared to sham which was set at zero. Local edema can occur after mTBI. Severe cerebral edema is associated with poor outcomes including increased mortality after mTBI with Second Impact Syndrome (2). *Denotes significance at p<0.05, n=6
| S-6 |
Figure 3
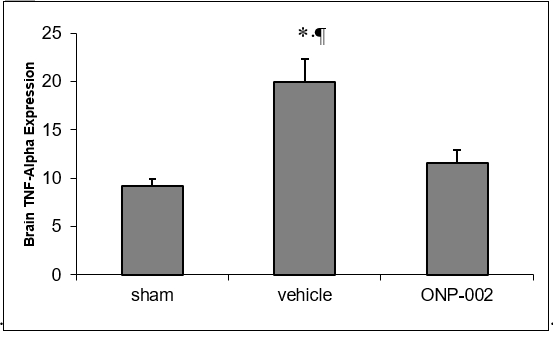
Inflammation. mTBI causes vascular and neuronal stress. Microglia and reactive astrocytes infiltrate the areas of injury and release inflammatory mediators, like TNF-alpha (3). In Figure 3, we show that ONP-002 (4mg/kg) reduces TNF-alpha-mediated neuroinflammation in brain tissue of rats compared to vehicle at 24-hrs after mTBI (ELISA). *denotes significance at p<0.05, n=6
Figure 4
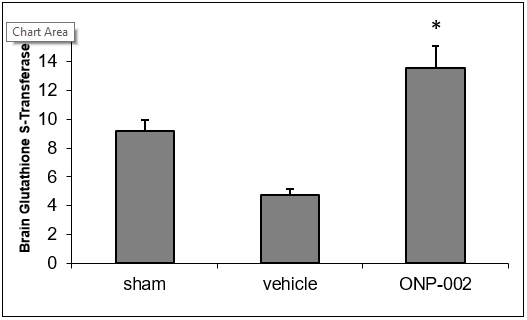
Oxidative Stress. Brain trauma causes Diffuse Axonal Injury (DAI) leading to elevated production of Reactive Oxygen Species (ROS), which causes neuronal damage (4). In Figure 4, we show that early treatment with ONP-002 (4mg/kg) improves antioxidant capacity in rats compared to vehicle-treated following mTBI by increasing protein expression of GST (ELISA) at 24hrs post-injury. *Denotes significance at p<0.05, n=6
| S-7 |
Figure 5
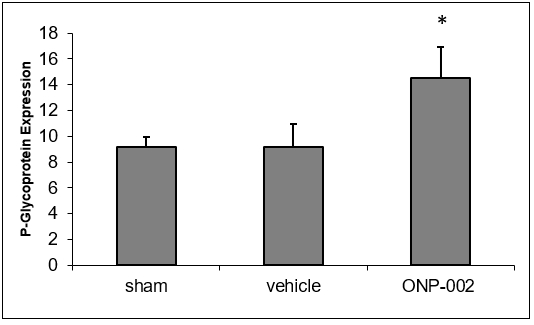
Autophagy. Brain trauma leads to neuronal damage with both intra and extracellular debris accumulation, which reduces cellular function and can lead to cell death (5). In Figure 5, we show that treatment with ONP-002 (4mg/kg) increases P-glycoprotein (PGP, ELISA), a molecule involved in macro-autophagy needed for intra and extracellular cleaning, in rats compared to vehicle-treated following mTBI at 24hr post-injury. *Denotes significance at p<0.05, n=6
Behavioral Studies
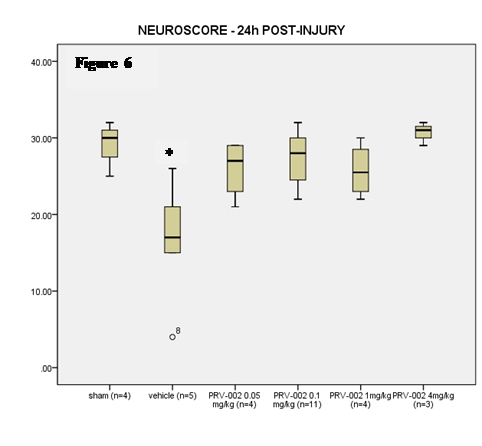
Neuroscore. In Figure 6, we show that treatment with ONP-002 (aka PRV-002) in rats enhances re-acquisition of motor skills by 24-hrs following mTBI when compared to vehicle-treated. Vestibular and visual motor dysfunction are hallmark signs of grade 3, severe mTBI where discoordination, dizziness and brain fog are seen and correlated to a greater incidence of Post-Concussion Syndrome (PCS) (6). A dose-response effect was seen with ONP-002 treatment at 4mg/kg producing the most desired effects. There were no statistical differences between ONP-002 treatments and sham. Data was analyzed using Kruskall-Wallis’s test to evaluate group differences. Pair-wise comparisons were carried out using the Mann-Whitney U Test. * denotes significance at p<0.05.
| S-8 |
Figure 7
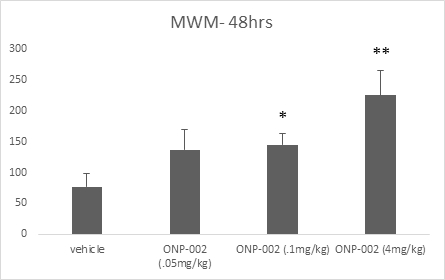
Short-Term Memory. Using the Morris Water Maze (MWM) method to assess shorter-term memory function we show in Figure 7 a dose-response effect at 48-hrs post-injury. ONP-002-treated rats had improved memory with significance seen between ONP-002 at 4mg/kg and vehicle-treated animals. Short-term memory deficits are often seen immediately following mTBI. Short-term memory impairment is a hallmark sign of PCS where deficits are seen for weeks or even longer (7). A one-way analysis of variance (ANOVA) was used to evaluate group differences in MWM memory score. Post-hoc analysis of pair-wise comparisons was carried out using Fisher’s Protected Least Significant Differences (PLSD) test. * denotes significance from vehicle at p<0,05, **denotes significance at p<0.005 from all groups. n=11
Figure 8
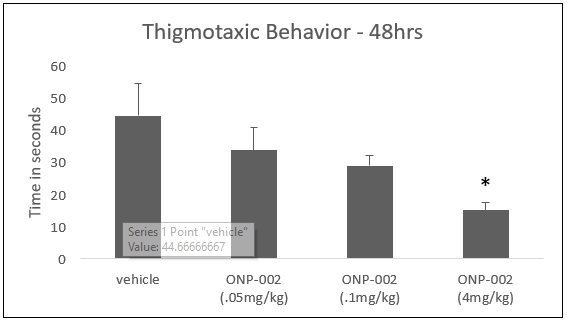
Thigmotaxic Behavior. Using the MWM tank to assess depression/anxiety-like behavior we show in Figure 8 a dose response effect on thigmotaxic behavior and that ONP-002 (4mg/kg)-treated rats had reduced thigmotaxis at 48-hrs post-injury compared to vehicle-treated. Depression and anxiety are commonly seen in patients diagnosed with PCS (8). ANOVA was used to evaluate group differences in thigmotaxis. * Denotes significance at p<0.005, n=8
| S-9 |
Cell Culture Studies
Figure 9
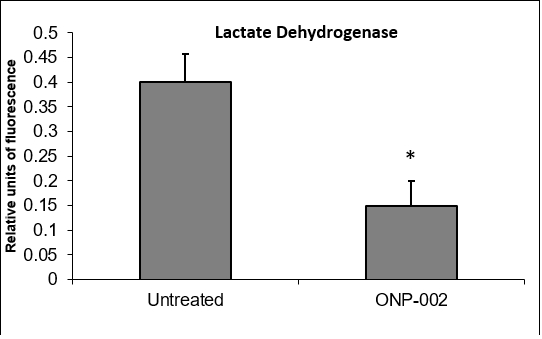
Neuronal Cell Viability. In a neuronal cell culture model (SH-SY5Y) of hypoxia-ischemia (oxygen-glucose deprivation (OGD), 24hrs), ONP-002 (5μM) reduces cell damage (Figure 9) as a measure of lactate dehydrogenase (membrane disruption) in cultured media. * Denotes significance at p<0.005
Figure 10
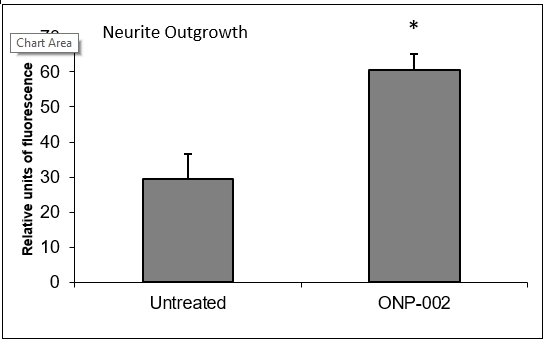
Neuronal Cell Recovery. Neurite outgrowth, indicative of cellular health and re-connectivity, was enhanced with the administration of ONP-002 (1μM) following exposure to hypoxia-ischemia (OGD) for 24hrs in a SH-SY5Y neurons compared to no treatment in cultured media.
| S-10 |
Figure 11
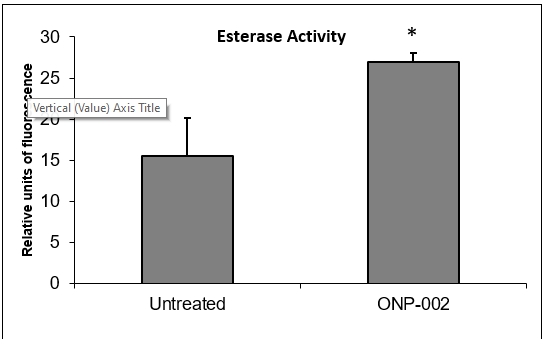
Neuronal Survival. Significant increases in esterase activity (Figure 11), a measure of cell viability, were observed in SH-SY5Y neurons treated with ONP-002 (1µM) following 24hrs of OGD in cultured media. * Indicates a significant difference from untreated OGD cells, p < 0.05.
Brain Biodistribution Studies. Table 2 below represents a comparison of ONP-002 levels in various parts of the brain, blood plasma and cerebrospinal fluid (CSF) in dogs (n=3) that were dosed intranasal (IN) 3 times; time 0, 4-hr and 8-hr. Dogs were sacrificed, and tissues were harvested at 30 minutes after the 8-hr final dose. Using a micro atomizer, dogs were given 23mg in each nostril dissolved in a 1ml of 22.5% HPβCD vehicle solution at each dosing interval for a total dose of 46 mg/dog per dosing interval. IN delivery increases direct drug flow into the brain in shorter periods of time through the peri axonal space of the olfactory nerves that innervate the superior chambers of the nasal cavity. The utility of IN dosing as a clinical route to achieve greater drug targeting of the brain and less systemic exposure was confirmed in this study whereby IN dosing of ONP-002 achieved up to 3.1-fold higher exposure in whole brain tissue (1888 ng/g) compared to systemic plasma (607 ng/ml). Further supporting the IN route of delivery these results show a total of 93% of the recovered drug was found in the brain. Only 5% of the drug was found in the circulating plasma and only 2% was found in the CSF. Comparable levels of ONP-002 were found throughout the brain at 30-min after the 8-hr treatment.
Table 2. Comparative exposure of ONP-002 in brain tissue, CSF and plasma following IN dosing in dogs
| Tissue | Subregion | Mean_ONP-002 concentration (ng/g brain) or (ng/ml CSF and plasma) |
Fold difference tissue exposure/plasma exposure | |||
| Brain tissue section | Frontal lobe | 2403 | 3.9 | |||
| Occipital lobe | 2332 | 3.8 | ||||
| Olfactory lobe | 2049 | 3.4 | ||||
| Parietal lobe | 2386 | 3.9 | ||||
| Temporal lobe | 2368 | 3.9 | ||||
| Whole brain | 1888 | 3.1 | ||||
| CSF | 33.2 | 0.05 | ||||
| Plasma | 607 | 1 | ||||
| S-11 |
ONP-002 Toxicology and Safety Program
Early Work in Pharmacokinetics. ONP-002 was evaluated in IND-enabling studies to uncover any potential safety liabilities associated with this treatment. Initially, several dose-range studies were performed in rats to determine the maximum tolerated systemic dose and to compare relative exposure/tolerability following multiple routes of administration, including the route intended for human dosing which is IN. These studies were used to guide dosing for the pivotal (GLP) 14-day repeat dose toxicology studies in dogs. A single and 7-day repeat dose of 38.5 mg/kg was well tolerated in rats and deemed to be the maximum tolerated dose (MTD). The resulting ONP-002 plasma exposure in these animals was very high (Cmax 120863 ng/ml), which exceeded exposure following IN administration of ONP-002 at a dose of 4.6 mg/kg (63.8 ng/ml), just above the dose of 4 mg/kg at which maximum efficacy was observed in the rat TBI model. Absorption following IN dosing was more rapid, as was clearance, with minimal drug evident in plasma by 24-hrs post dose compared to IV.
Pharmacokinetics and Safety of IN ONP-002 in Dog. This pivotal GLP 14-day study used repeat dosing of ONP-002, 3X a day, approximately 4 hours apart, for 14 consecutive days at concentrations of 0, 3, 10 or 23 mg/mL at a volume of 1 mL/nostril to beagle dogs (both nostrils had drug administered). The IN treatment was given as a liquid solution using a micro atomizer using 22.5% HPβCD as the vehicle. IN ONP-002 dosing revealed that ONP-002 was well tolerated up to the highest dose of 23 mg/ml or 46mg in total per dosing. Clinical observations were limited to increased salivation in dogs which occurred in a dose-dependent manner. There were no effects on body weight, food consumption, ophthalmic parameters, clinical chemistry, haematology, or organ weights at any of the doses tested. Microscopic analysis revealed purulent exudates in the nasal turbinate and evidence of inflammatory infiltrates and fibrin deposition in the lungs. All of these events were classified as mild, reversed during the recovery period, and did not appear to show any dose dependency. Similar findings were evident in vehicle control treated dogs indicating the findings were vehicle related. The highest dose of 23 mg/ml was thus determined to be the NOAEL which is equivalent to a ONP-002 dose of 1.5mg/kg and 2.3mg/kg in male and female dogs, respectively. Table 3 shows the dose-dependent increase in plasma exposure of ONP-002 in male and female dogs following IN administration. Plasma exposure levels were similar in males and females and there did not appear to be any evidence of drug accumulation following multiple doses.
| Table 3 - Dogs | Mean systemic exposure parameters | |||||||||||||
| Males (15kg weight) | Females (10kg weight) | |||||||||||||
| IN ONP-002 Dose concentration (mg/ml) | 3 | 10 | 23 (1.5mg/kg) | 3 | 10 | 23 (2.3mg/kg) | ||||||||
| Cmax (ng/ml) | D1 | 103 | 225 | 751 | 61.8 | 468 | 744 | |||||||
| D14 | 85.7 | 235 | 591 | 82.3 | 408 | 681 | ||||||||
| AUC (ng*h/ml) | D1 | 1100 | 2470 | 7410 | 804 | 3400 | 8260 | |||||||
| D14 | 1090 | 3490 | 9140 | 1010 | 5250 | 10100 | ||||||||
Cardiopulmonary Safety Pharmacology. The effect of ONP-002 on the human ether-a-go-go related gene (hERG) tail currents was assessed in a non-Good Laboratory Practice (GLP) study using manual whole-cell patch clamp (Table 4). ONP-002 tested at a single concentration of 10 μM inhibited hERG tail currents by 42.6% (n=3). In order to achieve a safety factor of 30-fold between in vitro hERG IC50 and free plasma levels of ONP-002 in clinical studies, Cmax should not exceed a free drug concentration of 0.33 μM (99 ng/ml). ONP-002 is 97.2% human plasma protein bound and is estimated to reach a plasma Cmax of 12.5 nM, the highest dose of 0.533 mg/kg to be administered in the planned first in human (FIH) study, which provides a safety factor of 800-fold. A GLP study is planned at Charles River, Inc. and will be performed prior to IND submission.
| S-12 |
Table 4. Inhibition of hERG tail currents by ONP-002
| Compound ID | Client compound ID |
Batch # | Concentration (μM) | % Inhibition | Mean | |||||||||
| N1 | N2 | N3 | ||||||||||||
| US034-0012558-1 | ONP-002 | 4 | 10 | 36.58 | 37.25 | 53.85 | 42.56 | |||||||
Positive reference control |
Cisapride | 1 | 96.99 | - | - | 96.99 | ||||||||
ONP-002 did not exert any effects on cardiac safety parameters evaluated by telemetry in male and female dogs as part of a 14-day repeat dose GLP toxicology study. Intranasal doses of 3, 10 and 23 mg/ml ONP-002 administered 3 times daily for 14 days did not have any significant effects on heart rate, pulse rate (PR), QRS and QT/QTc intervals or respiratory rate (RR) interval when compared to pre-dose values and vehicle control animals. An atrial depolarization was noted pre-dose in 1 animal which is occasionally observed in dogs and was not considered to be related to test article. The NOAEL for cardiac related effects in dogs was thus determined to be ≤ 23 mg/ml (equivalent to 4.8 mg/kg in male dogs and 7.2 mg/kg in female dogs). ONP-002 was also evaluated for potential central nervous system (CNS) toxicity in rats using a standard functional observational battery that was included as part of the 14-day repeat dose GLP toxicology study. IN administration of ONP-002 at 3, 10 and 23 mg/ml 3 times daily for 14 days was well tolerated with excess salivation and nares staining being the only observations occurring in 2 rats (one female in the 10 mg/ml group and 1 male in the 23 mg/ml group). Both incidences were not evident by the end of the 14-day recovery period.
As the intended clinical route of administration of ONP-002 is IN, the effects of ONP-002 on respiratory function were evaluated in a stand-alone GLP safety pharmacology study in male rats using whole-body plethysmography. ONP-002 administered as a single IN dose of 0.04, 0.4 or 4 mg/kg had no significant effect on respiratory rate, tidal volume (TV) or minute volume (MV) compared to vehicle treated animals and pre-dose values. In addition, there were no observations of respiratory distress following IN dosing of ONP-002 in repeat-dose GLP toxicology studies in rats and dogs. Tachypnea was however, observed in a dose range finding and 7-day repeat dose study in rats following intravenous (IV) administration of ONP-002, where systemic exposure of ONP-002 was almost 300-fold higher that that reached by the IN route.
Absorption, Distribution and Metabolism. Caco-2 permeability which was used as a surrogate for potential BBB permeability, was high with little evidence of PGP or breast cancer resistant protein mediated efflux. Cytochrome P450 mediated metabolism of ONP-002 is predominantly via CYP3A4. No significant inhibition, including time-dependent inhibition, was observed against major cytochrome P450 isoforms when ONP-002 was tested at a single concentration of 10 μM. There was some evidence of CYP2D6 and CYP3A4 induction (up to 3-fold) at a ONP-002 concentration of 100 μM. ONP-002 (10 μM) also exhibited minimal activity against a panel of major solute carriers and ATP-binding cassette (ABC) transporters, with inhibition of organic cation transporter 2 (OCT2) (89%) being the only result of note.
Genotoxicity. ONP-002 did not exhibit mutagenic activity in the bacterial AMES test or clastogenic activity in the mammalian in vitro micronucleus assay up to a maximum concentration of 100 μM. We have scheduled the In Vivo Micronucleus testing which is required for an IND with the FDA. We expect it will be completed by the end of Q3 2024.
Summary
| ● | The drug has completed toxicology studies in rats and dogs. Studies show that ONP-002 has a safety margin over 90X its predicted efficacious dose. | |
| ● | In preclinical animal studies, the asset demonstrated rapid and broad biodistribution throughout the brain while simultaneously reducing swelling, inflammation, and oxidative stress. | |
| ● | We believe ONP-002 has an equivalent, and potentially superior, neuroprotective effect compared to related neurosteroids. |
| ● | Animals treated with the ONP-002 post-concussion showed positive behavioral outcomes using various testing platforms including improved memory and sensory-motor performance, and reduced depression/anxiety like behavior. |
| S-13 |
ONP-002 Clinical Trials
ONP-002 has completed a Phase 1 clinical trial in healthy human subjects showing it is safe and well tolerated.
Safety studies have established a dosing regimen of 2X/day for fourteen days. The Phase I clinical trial was performed in Melbourne, Australia with a Contract Research Organization (CRO), Avance Clinical Pty Ltd and Nucleus Network Pty Ltd. The country of Australia provides a greater than 26% currency exchange advantage and a 43.5% rebate at the end of our fiscal year from the Australian government on all Research and Development performed in Australia. A comprehensive Investigator’s Brochure was created and approved by the Alfred Ethics Committee in Australia.
The Phase 1 study was double-blinded, randomized and placebo controlled (3:1, drug:placebo). Phase 1 used a Single Ascending/Multiple Ascending (SAD/MAD) drug administration design. The SAD component was a 1X treatment (low, medium, or high dose) and the MAD component was a 1X/day treatment for five consecutive days (low and medium dose). Blood and urine samples were collected at multiple time points for safety pharmacokinetics. Standard safety monitoring was provided for each body system.
Forty human subjects (31 males, 9 females) were successfully enrolled in Phase I. The Safety Review Board, made up of medical doctors, has reviewed the trial data and has determined the drug is safe and well tolerated at all dosing levels.
Oragenics anticipates preparing for Phase 2 clinical trials to further evaluate ONP-002’s safety and efficacy. Based on the Phase I data, we plan to apply for an Investigational New Drug application with the FDA and conduct a Phase II trial in the United States.
We anticipate a Phase 2 clinical trial will be performed administering ONP-002 intranasally in concussed patients 2x a day for up to fourteen days. The Phase 2a feasibility study will be performed in AUS with a target initiation date in the second quarter of 2024 to be followed closely by a Phase 2b proof of concept study in the US following IND approval in the fourth quarter of 2024.
Planned Phase 2a Feasibility study
| ● | n (40) – 20 patients per arm. | |
| ● | Two arms – Low dose or placebo/High dose or placebo. | |
| ● | Evaluating enrollment methods, safety and pharmacokinetics in concussed patients |
Planned Phase 2b Proof of concept (POC) with Early Efficacy
| ● | n (120) – 60 patients per arm. | |
| ● | One arm receives placebo – One arm receives highest safe dose from feasibility study. | |
| ● | POC measurements; blood biomarkers, neurocognitive and visual-vestibular measures, symptom severity and incidence of developing Post-Concussion Syndrome (PCS), time to return to normal activities. |
Product Candidates
| Product/Candidate | Description | Status | ||
| ONP-002 | Intranasal drug for the treatment of moderate-to-severe concussion in the acute through subacute phases (mTBI) | Completed Phase I | ||
| ONP-001(*) | Neurosteroid being developed for the treatment of Niemann Pick Type-C Disease (NPC) | Pre-clinical | ||
| NT-CoV2-1 | Intranasal vaccine candidate (plasmid + adjuvant) to provide long lasting immunity against SARS-CoV-2 | Pre-clinical | ||
| Antibiotics | For the treatment of healthcare-associated infections. Semi-synthetic analogs of MU1140: Member of lantibiotic class of antibiotics. | Pre-clinical – Inactive |
* We currently own only 50% of the intellectual property related to ONP-001. The other 50% is owned by a third party. We anticipate this product candidate will be developed through a joint venture with a third party. However, the joint venture with that third party has not been finalized.
| S-14 |
Recent Developments
On December 4, 2023, we filed a Certificate of Designation in the form of Articles of Amendment to our Articles of Incorporation to create the Series F Non-Voting Convertible Preferred Stock issued to Odyssey as partial consideration for the purchase of the Neurology Assets.
On December 14, 2023, as a result of the approval by our shareholders at our annual meeting of the increase in our authorized common stock, (i) we filed Articles of Amendment to increase our authorized shares of common stock to 350,000,000, and (ii) our Series E Preferred Stock expired and is no longer issued and outstanding.
On December 28, 2023, we successfully consummated our previously announced Asset Purchase Agreement with Odyssey, pursuant to which we purchased all of Odyssey’s assets (and assumed certain related liabilities) related to the segment of Odyssey’s business focused on developing medical products that treat brain related illnesses and diseases (the “Neurology Assets”). The Neurology Assets include drug candidates for treating mild traumatic brain injury (mTBI), also known as concussion, and for treating Niemann Pick Disease Type C (NPC), as well as Odyssey’s proprietary powder formulation and its nasal delivery device.
We have made several changes to reduce cash used in operations until additional capital can be obtained. As previously announced, we recently exercised our option under our lease with Hawley-Wiggins, LLC (the “Landlord”), for the building located in Progress Park and known as 13700 Progress Boulevard, Alachua, Florida 32615 (the “Lease”) to terminate the Lease by paying nine (9) months of advance rent, plus prorated rent for the month of September, 2023, plus applicable sales tax. In addition to the termination of the Lease, the Company eliminated two staff positions and Dr. Martin Handfield transitioned from an employee of the Company to a consultant. Dr. Handfield continues to be available to provide support services on an hourly basis through a consulting agreement. Dr. Handfield’s employment agreement was terminated in accordance with its terms. The Alachua lease contained the laboratory where some of the research and development for the lantibiotic program was undertaken. Currently, research and development activities related to the lantibiotic program are inactive. We intend to evaluate opportunities for this program moving forward as we continue to strengthen our focus and expertise on our intranasal drug delivery platform and drug candidates.
Corporate and Other Information
We were incorporated in November 1996 and commenced operations in 1999. We consummated our initial public offering in June 2003. Our executive office is located at, 4902 Eisenhower Boulevard, Suite 125 Tampa, Florida, 33634 and our research facilities are located at 13700 Progress Boulevard, Alachua, Florida 32615. Our telephone number is (813) 286-7900 and our website is http://www.oragenics.com. We make available free of charge on our website our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports as soon as reasonably practicable after we electronically file or furnish such materials to the Securities and Exchange Commission (the “SEC”). The reports are also available at www.sec.gov. We do not incorporate by reference into this prospectus the information on, or accessible through, our website, and you should not consider it as part of this prospectus and it should not be relied on in connection with this offering. We have included our website address as an inactive textual reference only.
| S-15 |
Before purchasing our securities you should carefully consider the risk factors set forth below and under the heading “Risk Factors” included in our most recent Annual Report on Form 10-K as revised or supplemented by our subsequent Quarterly Reports on Form 10-Q, each of which are on file with the SEC and are incorporated herein by reference, as well as all other information contained in this prospectus supplement and the accompanying prospectus and incorporated by reference and any free writing prospectus that we have authorized for use in connection with this offering. The risks and uncertainties described below and in our most recent Annual Report on Form 10-K, as revised or supplemented by our subsequent Quarterly Reports on Form 10-Q, are not the only risks and uncertainties we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business operations. Our business, financial condition and results of operations could suffer as a result of these risks. As a result, the trading price of our stock could decline, and you could lose all or part of your investment. The risks discussed below and in most recent Annual Report on Form 10-K, as revised or supplemented by our subsequent Quarterly Reports on Form 10-Q, also include forward-looking statements and our actual results may differ substantially from those discussed in these forward-looking statements. See the section entitled “Forward-Looking Information”.
Risk Related to Purchase of Neurology Assets from Odyssey
We may have difficulty raising additional capital, which could deprive us of the resources necessary to implement our business plan, which would adversely affect our business, results of operation and financial condition.
We need to raise additional capital to fund the development and commercialization of our product candidates and to operate our business, including ONP-002. The need to raise additional capital is expected to increase as a result of our purchase or the Neurology Assets. Part of the purchase price we paid Odyssey is $1,000,000 in cash. Additionally, we expect our operating expenses to increase, both due to additional employment costs and operating costs required to pursue the development of the Neurology Assets. In order to support the initiatives envisioned in our business plan, we will need to raise additional funds through the sale of assets, public or private debt or equity financing, collaborative relationships or other arrangements. If our operations expand faster or at a higher rate than currently anticipated, we may require additional capital sooner than we expect. We are unable to provide any assurance or guarantee that additional capital will be available when needed by our company or that such capital will be available under terms acceptable to our company or on a timely basis.
Our ability to raise additional financing depends on many factors beyond our control, including the state of capital markets, the market price of our common stock and the development or prospects for development of competitive products by others. If additional funds are raised through the issuance of equity, convertible debt or similar securities of our company, the percentage of ownership of our company by our company’s stockholders will be reduced, our company’s stockholders may experience additional dilution upon conversion, and such securities may have rights or preferences senior to those of our common stock. The preferential rights granted to the providers of such additional financing may include preferential rights to payments of dividends, super voting rights, a liquidation preference, protective provisions preventing certain corporate actions without the consent of the fund providers, or a combination thereof. We are unable to provide any assurance that additional financing will be available on terms favorable to us or at all.
If adequate funds are not available or are not available on acceptable terms, our ability to take advantage of the Neurology Assets acquired from Odyssey will be limited significantly. With limited capital, we expect to continue to scale back or delay implementation of research and development of our Covid programs and to put our lantibiotics on hold, and we may choose instead to focus the limited capital on the concussion asset purchased from Odyssey. Thus, the unavailability of capital could substantially harm our business, results of operations and financial condition.
| S-16 |
Our success with regard to the Neurology Assets depends on the viability of our business strategy with regard to those assets, which is unproven and may be unfeasible.
Our revenue and income potential with regard to the Neurology Assets, in particular the concussion asset, are unproven, and we continue to develop our strategy for such assets. Our anticipated business model is based on a variety of assumptions based on a growing trend in the healthcare systems in the United States and many other countries. These assumptions may not reflect the business and market conditions we actually face. As a result, our operating results could differ materially from those projected under our business model, and our business model may prove to be unprofitable. The product candidate ONP-002 (the concussion asset) being developed is in its early stages and will require extensive testing and clinical trials before it is commercialized. There is no guarantee that ONP-002 will be approved for commercial use. The product candidate ONP-001 (the potential treatment for Niemann Pick Disease Type C) is in its early stages and will require extensive testing and clinical trials before it is commercialized. There is no guarantee that ONP-001 will be approved for commercial use. Further, we own 50% of the the rights to this product candidate, with the other 50% owner by a third party. We anticipate this product candidate will be developed through a joint venture with a third party. However, the joint venture with that third party has not been finalized. If we fail to obtain marketing authorization for these product candidates, our business, financial condition, and results of operations will be materially adversely affected.
There are substantial inherent risks in attempting to commercialize newly developed products, and, as a result, we may not be able to successfully develop the new products acquired from Odyssey.
We hope to conduct research and development of the purchased Neurology Assets. However, commercial feasibility and acceptance of such product candidates are unknown. Scientific research and development require significant amounts of capital and takes an extremely long time to reach commercial viability, if at all. During the research and development process, we may experience technological barriers that we may be unable to overcome. Because of these uncertainties, it is possible that some or all of our future product candidates will never be successfully developed. If we are unable to successfully develop new products, we may be unable to generate new revenue sources or build a sustainable or profitable business. Additionally, as a result of the Odyssey transaction, since we operate with limited resources and staff, our attention and resources will be diverted away from our existing lantibiotic and Covid programs, resulting in further delays in the development and commercialization of such programs.
We will need to achieve commercial acceptance of our products, if cleared or approved, to generate revenues and achieve profitability.
Superior products may be introduced that compete with the Neurology Assets, which would diminish or extinguish the uses for the products candidates acquired from Odyssey, if cleared or approved. We cannot predict when significant commercial market acceptance for such products, if cleared or approved, will develop, if at all, and we cannot reliably estimate the projected size of any such potential market. If markets fail to accept such products, then we may not be able to generate revenue from them. Our revenue growth and achievement of profitability will depend substantially on our ability to introduce new products that are accepted by customers. Our competitors in the industry are predominantly large companies with longer operating histories, with significantly easier access to capital and other resources and an established product pipeline than us. There can be no assurance that we will be able to establish ourselves in our targeted markets, or, if established, that we will be able to maintain our market position, if any. Our commercial opportunity may be reduced if our competitors develop new or improved products that are more convenient, more effective or less expensive than our product candidates are. Competitors also may obtain FDA or other regulatory marketing authorization for their products more rapidly or earlier than we may obtain marketing authorization for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. If we are unable to cost-effectively achieve acceptance of our products by customers, or if our products do not achieve wide market acceptance, then our business will be materially and adversely affected.
The products candidates included in the Neurology Assets are still in development and we have not obtained authorization from any regulatory agency to commercially distribute such products in any country and we may never obtain such authorizations.
We currently have no products authorized for commercial distribution in either the United States, Europe or any other country. Similarly, the products candidates we acquired from Odyssey are still in development. Like the product candidates we are developing, the Neurology Assets require regulatory clearance or approvals. We cannot begin marketing and selling product candidates until we obtain applicable authorizations from the applicable regulatory agencies. The process of obtaining regulatory authorization is expensive and time-consuming and can vary substantially based upon, among other things, the type, complexity and novelty of a product candidate. Changes in regulatory policy, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application may cause delays in the authorization of a product candidate or rejection of a regulatory application altogether.
| S-17 |
The FDA has substantial discretion in the review process and may refuse to accept our application or may decide that data are insufficient to grant the request and require additional pre-clinical, clinical, or other studies. In addition, varying interpretations of the data obtained from pre-clinical and clinical testing could delay, limit, or prevent marketing authorization from the FDA or other regulatory authorities. Any marketing authorization from the FDA we ultimately obtain may be limited or subject to restrictions or post-market commitments that render the product candidate not commercially viable. If our attempts to obtain marketing authorization are unsuccessful, we may be unable to generate sufficient revenue to sustain and grow our business, and our business, financial condition, and results of operations will be materially adversely affected.
We are, and will continue to be, dependent in significant part on outside scientists and third-party research institutions for our research and development in order to be able to commercialize our product candidates.
We currently have a limited number of employees and resources available to perform the research and development necessary to commercialize our product candidates and potential future product candidates. We therefore rely, and will continue to rely, on third-party research institutions, collaborators and consultants for this capability. While the Company continues to seek additional funding, it is taking steps to reduce the use of its cash resources, which include the determination to terminate the Lease.
We are heavily dependent upon the ability and expertise of our management team and a very limited number of employees and the loss of such individuals could have a material adverse effect on our business, operating results or financial condition.
We currently have a very small management team. Our success is dependent upon the ability, expertise and judgment of our senior management. While employment agreements are customarily used as a primary method of retaining the services of key employees, these agreements cannot assure the continued services of such employees. Any loss of the services of such individuals could have a material adverse effect on our business, operating results or financial condition.
We believe that our future success with regard to the Neurology Assets will depend significantly on the skills and efforts of Joseph Michael Redmond, our new President, and other key personnel, including Jacob VanLandingham, Ph.D., one of the Company’s independent contractors. The loss of the services of any of these individuals could harm our ability to successfully pursue the development of the Neurology Assets. If any of our executive officers or key personnel left or was otherwise unable to work and we were unable to find a qualified replacement and/or to obtain adequate compensation for such loss, we may be unable to manage our business, which could harm our operating results and financial condition.
Mr. Redmond continues to serve as President and CEO of Odyssey, which may give rise to conflicts of interest, including with regard to the transaction between the Company and Odyssey related to the Neurology Assets. Additionally, while we expect Mr. Redmond to devote substantially all of his business time and efforts to the Company, we can make no assurance of this given his continuing role with Odyssey.
Prevacus, Inc., is the Company from whom Odyssey purchased the Neurology Assets in 2021. On December 5, 2022, the Mississippi Department of Human Services (“MDHS”) filed an Amended Complaint in the Circuit Court of Hinds County, Mississippi First Judicial District against Mississippi Community Education Center, Inc., a non-profit corporation, Nancy New, its director, Prevacus, Dr. VanLandingham its founder , and several other defendants, alleging, among other things, a conspiracy to defraud the MDHS. The MDHS is designated by Mississippi law as the State agency exclusively responsible for administering the federally-authorized and federally-funded anti-poverty (or “welfare”) program known as the Temporary Assistance for Needy Families program, or “TANF.” With regard to Prevacus and Dr. VanLandingham, the complaint alleges that $2.1 million was funneled through the Mississippi Community Education Center, a nonprofit run by Nancy New, to Prevacus and PresolMD, another company founded by Dr. VanLandingham. The MDHS, among other things, is seeking to recover $2.1 million it alleges went to Prevacus and PresolMD. Prevacus and Dr. VanLandingham have denied any wrongdoing and have denied being aware that the funds received from Community Education Center, Inc. were TANF funds. When Odyssey agreed to purchase the Prevacus assets, it did not agree to assume any liability for refunding MDHS. Similarly, any such liabilities would be deemed excluded liabilities under the Company’s asset purchase agreement with Odyssey. As such, the Company does not believe it has any financial exposure related to the reimbursement of the funds paid to Prevacus and does not believe there are any grounds on which the Company could become embroiled in the foregoing legal proceedings. Dr. VanLandinham is not an Officer or Diretcor of Oragenics, but is instead an independent contractor. Nevertheless, any negative media related to foregoing legal proceedings, and in particular any negative media related to the concussion assets or Dr. VanLandingham, may negatively impact the Company’s ability to raise capital and otherwise continue the development of the Neurology Assets. Furthermore, Dr. VanLandingham’s ability to continue to assist in the development of the Neurology Assets may be negatively impacted by his need to respond to, and participate in, the foregoing legal proceedings.
| S-18 |
As a result of the purchase of the Neurology Assets, we anticipate growth in our business and increased costs, and any inability to manage such growth could harm our business.
Our success will depend, in part, on our ability to effectively manage our growth and expansion. Any growth in, or expansion of, our business is likely to continue to place a significant strain on our management and administrative resources, infrastructure and systems. In order to succeed, we will need to continue to implement management information systems and improve our operating, administrative, financial and accounting systems and controls. We will also need to train new employees and maintain close coordination among our executive, accounting, finance and operations organizations. These processes are time-consuming and expensive, will increase management responsibilities and will divert management attention. Our inability or failure to manage our growth and expansion effectively could substantially harm our business and adversely affect our operating results and financial condition.
The third party upon whom we rely for the supply of ONP-002 is our sole source of supply, and the loss of this supplier could significantly harm our business.
ONP-002 is a fully synthetic, non-naturally occurring neurosteroid. Pharmaron, Inc. is our current partner in the development of synthetic chemistry and manufacturing of the ONP-002 (Molecular Formula: C20H28O2, Molecular Weight: 300.14 g/mol). Our ability to successfully develop our ONP-002 product candidates, and to ultimately supply our commercial products in quantities sufficient to meet the market demand, depends in part on our ability to obtain the drug product and drug substance for our product candidates in accordance with regulatory requirements and in sufficient quantities for commercialization and clinical testing. We do not currently have arrangements in place for a redundant or second-source supply of any products or substances in the event our current supplier ceases their operations or stops offering us sufficient quantities of these materials for any reason.
We are not certain that our single-source supplier will be able to meet our demand, either because of the nature of our agreement with the supplier, our limited experience with the supplier or our relative importance as a customer to the supplier. It may be difficult for us to assess its ability to timely meet our demand in the future based on past performance. While our supplier has generally met our demand on a timely basis in the past, they may subordinate our needs in the future to their other customers.
Moreover, if there is a disruption to our third-party manufacturers’ or suppliers’ relevant operations the supply of ONP-002 and its components will be delayed until such manufacturer or supplier restores the affected facilities or we or they procure alternative manufacturing facilities or sources of supply. Our ability to progress our pre-clinical and clinical programs could be materially and adversely impacted if any of the third-party suppliers upon which we rely were to experience a significant business challenge, disruption or failure due to issues such as financial difficulties or bankruptcy, issues relating to other customers such as regulatory or quality compliance issues, or other financial, legal, regulatory or reputational issues. Additionally, any damage to or destruction of our third-party manufacturers’ or suppliers’ facilities or equipment may significantly impair our ability to manufacture our product candidates on a timely basis.
Establishing additional or replacement suppliers for drug product and drug substance used in our product candidates, if required, may not be accomplished quickly and can take several years, if at all. Furthermore, despite our efforts, we may be unable to procure a replacement supplier or do so on commercially reasonable terms, which could have a material adverse impact upon our business. If we are able to find a replacement supplier, such replacement supplier would need to be qualified and may require additional regulatory approval, which could result in further delay. While we seek to maintain adequate inventory of the drug product and drug substance used in our product candidates, any interruption or delay in the supply of components or materials, or our inability to obtain such drug product and drug substance from alternate sources at acceptable prices in a timely manner could impede, delay, limit or prevent our development efforts, which could harm our business, results of operations, financial condition and prospects.
| S-19 |
Certain of the raw materials required in the manufacture and the formulation of our product candidates are derived from biological sources. Such raw materials are difficult to procure and may be subject to contamination or recall. Access to and supply of sufficient quantities of raw materials which meet the technical specifications for the production process is challenging, and often limited to single-source suppliers. Finding an alternative supplier could take a significant amount of time and involve significant expense due to the nature of the products and the need to obtain regulatory approvals. If we or our manufacturers are unable to purchase the raw materials necessary for the manufacture of our product candidates on acceptable terms in a timely manner, at sufficient quality levels, or in adequate quantities, if at all, our ability to produce sufficient quantities of our products for clinical or commercial requirements would be negatively impacted. A material shortage, contamination, recall or restriction on the use of certain biologically derived substances or any raw material used in the manufacture of our products could adversely impact or disrupt manufacturing, which would impair our ability to generate revenues from the sale of such product candidates, if approved or cleared.
If Odyssey were to convert all of its Series F Convertible Preferred Stock, they would own more than a majority of our outstanding shares of common stock.
At the closing of the Odyssey transaction, we issued 8,000,000 shares of Series F Convertible Preferred Stock to Odyssey, which are convertible into our common stock on a one-for-one basis. The Series F Convertible Preferred Stock is non-voting, but if Odyssey were to convert all of its shares of Series F Convertible Preferred Stock into our common stock, they would control the vote of more than a majority of our outstanding common stock. Such a conversion would likely be considered a change of control under the rules of the NYSE American, requiring us to apply for and meet the NYSE Americans initial listing standards. We do not currently meet those standards. Accordingly, our Certificate of Designation creating the Series F Preferred Stock specifies that the remainder of the Series F Convertible Preferred shares are not convertible until the occurrence of all of the following: (i) Oragenic’s shall have applied for and been approved for initial listing on the NYSE American or another national securities exchange or shall have been delisted from the NYSE American, which Oragenic’s does not anticipate undertaking until it meets the NYSE American’s initial listing standards, and (ii) if required by the rules of the NYSE American, Oragenic’s shareholders shall have approved any change of control that could be deemed to occur upon the conversion of the Series F Preferred Stock into common stock, based on the fact and circumstances existing at such time.
Risks Related To Our Financial Condition and Need For Additional Capital
We have incurred significant losses since our inception and expect to continue to experience losses for the foreseeable future.
We have incurred significant net losses and negative cash flow in each year since our inception, including net losses of approximately and $7.9 million and $12.1 million for the nine months ended September 30, 2023 and September 30, 2022, respectively, and approximately $14.2 million and $15.7 million for the years ended December 31, 2022, and 2021, respectively. As of September 30, 2023, our accumulated deficit was approximately $193.5 million. We have devoted a significant amount of our financial resources to research and development, including our nonclinical development activities and clinical trials. Additionally, in connection with the purchase of the Neurology Assets, we paid Odyssey $1.0 million. We expect that the costs associated with our plans to begin Phase 2 work on ONP-002 and our preclinical research for our NT-CoV2-1 vaccine product candidate, as well as any research and development of our Covid product candidate. Additionally, our License Agreements also require the payment of certain recurring and performance-based royalties that may negatively impact our financial capabilities. As a result, we expect to continue to incur substantial net losses and negative cash flow for the foreseeable future. These losses and negative cash flows have had, and will continue to have, an adverse effect on our shareholders’ equity and working capital. Because of the numerous risks and uncertainties associated with product development and commercialization, we are unable to accurately predict the timing or amount of substantial expenses or when, or if, we will be able to generate the revenue necessary to achieve or maintain profitability.
We will need to raise additional capital in the future to complete the development and commercialization of our product candidates and operate our business.
Developing and commercializing biopharmaceutical products, including Phase 2 work for our ONP-002 product candidate and conducting nonclinical studies and clinical trials and establishing manufacturing capabilities, and the progress of our efforts to develop and commercialize our product candidates, is expensive, and can cause us to use our limited, available capital resources faster than we currently anticipate. We anticipate that our estimated cash resources of approximately $3.5 million as of December 31, 2023, will be sufficient to fund our operations as presently structured through the second quarter of 2024. We are currently evaluating cost-saving initiatives, including restructuring that could allow further cash runway through 2024 to the extent such initiatives are undertaken. Our auditor has previously expressed substantial doubt about our ability to continue as a going concern and absent additional financing we may be unable to remain a going concern. Our actual costs may ultimately vary from our current expectations, which could materially impact our use of capital and our forecast of the period of time through which our financial resources will be adequate to support our operations. Our current cash, cash equivalents and short-term investments are not sufficient to fully implement our business strategy and sustain our operations. Accordingly, we will need to seek additional sources of financing and such additional financing may not be available on favorable terms, if at all. Until we can generate a sufficient amount of product revenue, if ever, we expect to finance future cash needs through public or private equity offerings, debt financings or corporate or government collaboration and licensing arrangements. If we do not succeed in raising additional funds on acceptable terms, we may be unable to complete existing nonclinical and planned clinical trials or obtain approval of our product candidates from the FDA and other regulatory authorities. We expect capital outlays and operating expenditures to increase over the next several years as we expand our infrastructure, and research and development activities. Specifically, we need to raise additional capital to, among other things:
| ● | conduct Phase 2 clinical trials for our ONP-002 product candidate; |
| S-20 |
| ● | conduct preclinical research for our NT-CoV-2-1 vaccine product candidate, file an IND with the FDA and, if approved, engage in Phase 1 clinical trials; | |
| ● | engage in GMP and non-GMP manufacturing for our product candidates at the preclinical research and clinical trial stages; | |
| ● | fund our clinical validation study activities; | |
| ● | expand our research and development activities; and | |
| ● | finance our capital expenditures and general and administrative expenses. |
Our present and future funding requirements will depend on many factors, including:
| ● | the level of research and development investment budgeted to develop our current and future product candidates through each phase of development; | |
| ● | the timing, scope, progress, results and cost of research and development, testing, screening, manufacturing, preclinical and non-clinical studies and clinical trials, including any impacts related to the COVID-19 pandemic; | |
| ● | costs of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights; | |
| ● | our need or decision to acquire or license complementary technologies or acquire complementary businesses; | |
| ● | changes in test development plans needed to address any difficulties in product candidate selection for commercialization; | |
| ● | competing neurological, vaccine and technological and market developments; | |
| ● | our interaction and relationship with the FDA, or other, regulatory agencies; and | |
| ● | changes in regulatory policies or laws that affect our operations. |
Additional capital may not be available on satisfactory terms, or at all. Furthermore, if we raise additional funds by issuing equity securities, dilution to our existing stockholders could result. Any equity securities issued also may provide for rights, preferences or privileges senior to those of holders of our common and preferred stock. If we raise additional funds by issuing debt securities, these debt securities would have rights, preferences and privileges senior to those of holders of our common stock, and the terms of the debt securities issued could impose significant restrictions on our operations. If we raise additional funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our technologies or our products under development or grant licenses on terms that are not favorable to us, which could lower the economic value of those programs to us. If adequate funds are not available, we may have to scale back our operations or limit our research and development activities, which may cause us to grow at a slower pace, or not at all, and our business could be adversely affected.
In addition, we could be forced to discontinue product development and commercialization of one or more of our product candidates, curtail or forego sales and marketing efforts, and/or forego licensing attractive business opportunities.
| S-21 |
Risks Relating to this Offering
The market price of our common stock has been, and may continue to be volatile and fluctuate significantly, which could result in substantial losses for investors.
The trading price for our common stock has been, and we expect it to continue to be, volatile. The price at which our common stock trades depends upon a number of factors, including our historical and anticipated operating results, our financial situation, announcements by us or our competitors, our ability or inability to raise the additional capital we may need and the terms on which we raise it, and general market and economic conditions. Some of these factors are beyond our control. Broad market fluctuations may lower the market price of our common stock and affect the volume of trading in our stock, regardless of our financial condition, results of operations, business or prospects. The closing price of our common stock as reported on the NYSE American had a high price of $9.00 and a low price of $2.62 in the 52-week period ended December 31, 2023 and a high price of $6.84 and a low price of $5.75 from January 1, 2024 through January 15, 2024. Among the factors that may cause the market price of our common stock to fluctuate are the risks described in this “Risk Factors” section and other factors, including:
| ● | results of preclinical and clinical studies of our product candidates or those of our competitors; | |
| ● | regulatory or legal developments in the U.S. and other countries, especially changes in laws and regulations applicable to our product candidates; | |
| ● | actions taken by regulatory agencies with respect to our product candidates, clinical studies, manufacturing process or sales and marketing terms; | |
| ● | introductions and announcements of new products by us or our competitors, and the timing of these introductions or announcements; | |
| ● | announcements by us or our competitors of significant acquisitions or other strategic transactions or capital commitments; | |
| ● | fluctuations in our quarterly operating results or the operating results of our competitors; | |
| ● | variance in our financial performance from the expectations of investors; | |
| ● | changes in the estimation of the future size and growth rate of our markets; | |
| ● | changes in accounting principles or changes in interpretations of existing principles, which could affect our financial results; | |
| ● | failure of our products to achieve or maintain market acceptance or commercial success; | |
| ● | conditions and trends in the markets we serve; | |
| ● | changes in general economic, industry and market conditions; | |
| ● | changes in legislation or regulatory policies, practices or actions; | |
| ● | the commencement or outcome of litigation involving our company, our general industry or both; | |
| ● | recruitment or departure of key personnel; | |
| ● | changes in our capital structure, such as future issuances of securities, redemption or conversion of preferred stock or the incurrence of additional debt; | |
| ● | actual or expected sales of our common stock by our stockholders; | |
| ● | acquisitions and financings; and | |
| ● | the trading volume of our common stock. |
In addition, the stock markets, in general, NYSE American and the market for biotech companies in particular, may experience a loss of investor confidence. Such loss of investor confidence may result in extreme price and volume fluctuations in our common stock that are unrelated or disproportionate to the operating performance of our business, financial condition or results of operations. These broad market and industry factors may materially harm the market price of our common stock and expose us to securities class action litigation. Such litigation, even if unsuccessful, could be costly to defend and divert management’s attention and resources, which could further materially harm our financial condition and results of operations.
| S-22 |
You will experience immediate and substantial dilution as a result of this offering and may experience additional dilution in the future.
You will incur immediate and substantial dilution as a result of this offering. After giving effect to the sale by us of shares offered in this offering at the public offering price of $ per share, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us, investors in this offering can expect an immediate dilution of approximately $ per share. The exercise of outstanding stock options and warrants and the conversion of our outstanding preferred stock may result in further dilution of your investment and, with regard to our Series F Convertible Preferred Stock, will result in a material further dilution of your investment. See “Dilution” below for a more detailed discussion of the dilution you will incur if you purchase our securities in the offering.
Our management team may invest or spend the proceeds of this offering in ways with which you may not agree or in ways which may not yield a significant return.
Our management will have broad discretion over the use of proceeds from this offering. We intend to use the net proceeds from this offering to fund a portion of our ONP-002 research and clinical trials, and for working capital and general corporate purposes. Our management will have considerable discretion in the application of the net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. The net proceeds may be used for corporate purposes that do not increase our operating results or enhance the value of our common stock.
The precise amount and timing of the application of these proceeds will depend upon a number of factors, such as the timing and progress of our research and development efforts, our funding requirements and the availability and costs of other funds. As of the date of this prospectus supplement, we cannot specify with certainty all of the particular uses for the net proceeds to us from this offering. Depending on the outcome of our efforts and other unforeseen events, our plans and priorities may change and we may apply the net proceeds of this offering in different manners than we currently anticipated.
The failure by our management to apply these funds effectively could harm our business, financial condition and results of operations. Pending their use, we may invest the net proceeds from this offering in short-term, interest-bearing instruments. These investments may not yield a favorable return to our stockholders.
Future sales of our common stock in the public market could cause our stock price to fall.
Sales of a substantial number of shares of our common stock, or the perception by the market that those sales could occur, could cause the market price of our common stock to decline or could make it more difficult for us to raise funds through the sale of equity in the future.
Future issuances of common stock could further depress the market for our common stock. We expect to continue to incur drug development and selling, general and administrative costs, and to satisfy our funding requirements, we will need to sell additional equity securities, which may include sales of significant amounts of common stock to strategic investors, and which common stock may be subject to registration rights and warrants with anti-dilutive protective provisions. The sale or the proposed sale of substantial amounts of our common stock or other equity securities in the public markets or in private transactions may adversely affect the market price of our common stock and our stock price may decline substantially. Our stockholders may experience substantial dilution and a reduction in the price that they are able to obtain upon sale of their shares. Also, new equity securities issued may have greater rights, preferences or privileges than our existing common stock. In addition, we have a significant number of shares of restricted stock, stock options and warrants outstanding. To the extent that outstanding stock options or warrants have been or may be exercised or other shares issued, investors purchasing our common stock in this offering may experience further dilution.
If we make one or more significant acquisitions in which the consideration includes stock or other securities, our stockholders’ holdings may be significantly diluted. In addition, stockholders’ holdings may also be diluted if we enter into arrangements with third parties permitting us to issue shares of common stock in lieu of certain cash payments upon the achievement of milestones.
| S-23 |
The issuance of shares of our common stock under our 2021 Equity Incentive Plan is covered by Form S-8 registration statements we filed with the Securities and Exchange Commission, or SEC, and upon exercise of the options, such shares may be resold into the market. We have also issued shares of common stock and warrants in connection with previous private placements. Such shares are available for resale as well as certain of the shares of common stock issuable upon exercise of the warrants. We have also issued shares of our common stock in the private placement and financing transaction, which are deemed to be “restricted securities,” as that term is defined in Rule 144 promulgated under the Securities Act of 1933, as amended, or Securities Act, and such shares may be resold pursuant to the provisions of Rule 144. The resale of shares acquired from us in private transactions could cause our stock price to decline significantly. In addition, the conversion of outstanding shares preferred stock into common stock and the subsequent sale of shares of common stock could also cause our stock price to decline significantly.
In addition, from time to time, certain of our shareholders may be eligible to sell all or some of their restricted shares of common stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144, subject to certain limitations. In general, pursuant to Rule 144, after satisfying a six-month holding period: (i) affiliated shareholders, or shareholders whose shares are aggregated, may, under certain circumstances, sell within any three-month period a number of securities which does not exceed the greater of 1% of the then-outstanding shares of common stock or the average weekly trading volume of the class during the four calendar weeks prior to such sale and (ii) non-affiliated shareholders may sell without such limitations, in each case provided we are current in our public reporting obligations. Rule 144 also permits the sale of securities by non-affiliates that have satisfied a one-year holding period without any limitation or restriction.
We are unable to estimate the number of shares that may be sold because this will depend on the market price for our common stock, the personal or business circumstances of sellers and other factors.
We do not intend to pay cash dividends.
We have not declared or paid any cash dividends on our common stock, and we do not anticipate declaring or paying cash dividends for the foreseeable future. Any future determination as to the payment of cash dividends on our common stock will be at our Board of Directors’ discretion and will depend on our financial condition, operating results, capital requirements and other factors that our Board of Directors considers to be relevant.
You may experience future dilution as a result of future equity offerings.
To raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that may not be the same as the prices per share in this offering. We may sell shares or other securities in any other offering at a price per share that is less than the prices per share paid by investors in this offering, and investors purchasing shares of our common stock or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions may be higher or lower than the prices per share paid by investors in this offering.
We cannot assure you that we will continue to be listed on the NYSE American.
Our common stock commenced trading on the NYSE American (formerly the NYSE MKT) on April 10, 2013, and we are subject to certain NYSE American continued listing requirements and standards. We cannot provide any assurances that we satisfy and will continue to satisfy the NYSE American’s continued listing standards. A delisting of our common stock from the NYSE American could negatively affect the price and liquidity of our common stock and could impair our ability to raise capital in the future. We may incur costs that we have not previously incurred for expenses for compliance with the rules and requirements of the NYSE American. We may incur costs that we have not previously incurred for expenses for compliance with the rules and requirements of the NYSE American.
There is no established public trading market for the Pre-funded Warrants being offered in this offering, and we do not expect a market to develop for the Pre-funded Warrants.
There is no established public trading market for the Pre-funded Warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the Pre-funded Warrants on any national securities exchange or other nationally recognized trading system. Without an active market, the liquidity of the Pre-funded Warrants will be limited. Further, the existence of the Pre-funded Warrants may act to reduce both the trading volume and the trading price of our common stock.
| S-24 |
Except as otherwise provided in the Pre-funded Warrants, holders of Pre-funded Warrants purchased in this offering will have no rights as stockholders until such holders exercise their Pre-funded Warrants and acquire our common stock.
Except as otherwise provided in the Pre-funded Warrants, until holders of Pre-funded Warrants acquire our common stock upon exercise of the Pre-funded Warrants, holders of Pre-funded warrants will have no rights with respect to our common stock underlying such Pre-funded Warrants. Upon exercise of the Pre-funded Warrants, the holders will be entitled to exercise the rights of a holder of our common stock only as to matters for which the record date occurs after the exercise date.
We estimate that the net proceeds from this offering, after payment of estimated offering expenses payable by us and underwriting discounts will be approximately $ million. We intend to use the net proceeds from this offering to fund the continued development of our ONP-002 product candidate and for general corporate purposes and working capital.
The precise amount and timing of the application of these proceeds will depend upon a number of factors, such as the timing and progress of our research and development efforts, our funding requirements and the availability and costs of other funds. We have not determined the amounts we plan to spend on the areas listed above or the timing of these expenditures, and, as a result, as of the date of this prospectus supplement, we cannot specify with certainty all of the particular uses for the net proceeds to us from this offering. Accordingly, our management will have broad discretion in the timing and application of these proceeds. Pending application of the net proceeds as described above, we intend to temporarily invest the proceeds in short-term, interest-bearing instruments. Pending application of the net proceeds for the purposes as described above, we expect to invest the net proceeds in short-term, interest-bearing securities, investment grade securities, certificates of deposit or direct or guaranteed obligations of the U.S. government.
To date, we have neither declared nor paid any dividends on our common stock nor do we anticipate that such dividends will be paid in the foreseeable future. Rather, we intend to retain any earnings to finance the growth and development of our business. Any payment of cash dividends on our common stock in the future will be dependent, among other things, upon our earnings, financial condition, capital requirements and other factors which the board of directors deems relevant. In addition, restrictive covenants contained in any financing agreements entered into in the future may preclude us from paying any dividends.
DESCRIPTION OF PRE-FUNDED WARRANTS
The following is a brief summary of certain terms and conditions of the Pre-funded Warrants being offered in this offering. The following description is subject in all respects to the provisions contained in the Pre-funded Warrants.
Form
The pre-funded warrants will be issued as individual warrant agreements to the purchasers. The form of Pre-funded Warrant will be filed as an exhibit to a Current Report on Form 8-K that we will file with the SEC and is incorporated by reference in this prospectus supplement.
Term
The Pre-funded Warrants will not expire until they are fully exercised.
Exercisability
The Pre-funded Warrants are exercisable at any time until they are fully exercised. The Pre-funded Warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and payment of the exercise price. No fractional shares of common stock will be issued in connection with the exercise of a Pre-funded Warrant. The holder of the Pre-funded Warrant may also satisfy its obligation to pay the exercise price through a “cashless exercise,” in which the holder receives the net value of the Pre-funded Warrants in shares of common stock determined according to the formula set forth in the Pre-funded Warrant.
| S-25 |
Exercise Limitations
Under the terms of the Pre-funded Warrants, the Company may not effect the exercise of any such warrant, and a holder will not be entitled to exercise any portion of any such warrant, if, upon giving effect to such exercise, the aggregate number of shares of common stock beneficially owned by the holder (together with its affiliates, any other persons acting as a group together with the holder or any of the holder’s affiliates, and any other persons whose beneficial ownership of common stock would or could be aggregated with the holder’s for purposes of Section 13(d) or Section 16 of the Securities Exchange Act of 1934, as amended) would exceed 4.99% of the number of shares of common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of such warrant, which percentage may be increased or decreased at the holder’s election upon 61 days’ notice to the Company subject to the terms of such warrants, provided that such percentage may in no event exceed 9.99%.
Exercise Price
The exercise price of our shares of common stock purchasable upon the exercise of the Pre-funded Warrants is $0.001 per share. The exercise price of the Pre-funded Warrants and the number of shares of common stock issuable upon exercise of the Pre-funded Warrants is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our shares of common stock, as well as upon any distribution of assets, including cash, stock or other property, to our stockholders.
Transferability
Subject to applicable laws, the Pre-funded Warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing
We do not intend to list the Pre-funded Warrants on The NYSE American, any other national securities exchange or any other nationally recognized trading system.
Fundamental Transactions
Upon the consummation of a fundamental transaction (as described in the Pre-funded Warrants, and generally including any reorganization, recapitalization or reclassification of our shares of common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding shares of common stock, or any person or group becoming the beneficial owner of 50% of the voting power of our outstanding shares of common stock), the holders of the pre-funded warrants will be entitled to receive, upon exercise of the Pre-funded Warrants, the kind and amount of securities, cash or other property that such holders would have received had they exercised the pre-funded warrants immediately prior to such fundamental transaction, without regard to any limitations on exercise contained in the Pre-funded Warrants. Notwithstanding the foregoing, in the event of a fundamental transaction where the consideration consists solely of cash, solely of marketable securities or a combination of cash and marketable securities, then each Pre-funded Warrants shall automatically be deemed to be exercised in full in a cashless exercise effective immediately prior to and contingent upon the consummation of such fundamental transaction.
No Rights as a Stockholder
Except by virtue of such holder’s ownership of shares of common stock or as otherwise provided in the Pre-Funded Warrants, the holder of a Pre-funded Warrant does not have the rights or privileges of a holder of our shares of common stock, including any voting rights, until such holder exercises the Pre-funded Warrant.
| S-26 |
If you purchase shares of common stock in this offering, you will experience dilution to the extent of the difference between the public offering price of the shares of common stock in this offering and the net tangible book value per share of our common stock immediately after this offering.
As of September 30, 2023, our net tangible book value was $6,472,984, or $2.52 per share of our common stock, based upon 2,569,385 shares of common stock outstanding as of that date. Historical net tangible book value per share is equal to our total tangible assets minus total liabilities, all divided by the number of shares of common stock outstanding as of September 30, 2023. Dilution in net tangible book value per share represents the difference between the amount per share paid by purchasers of shares of common stock in this offering and the net tangible book value per share of common stock immediately after this offering.
Our adjusted net tangible book value, as of September 30, 2023, was $5,472,984, or $1.78 per share of our common stock based upon 3,080,693 shares of common stock outstanding as of that date. Adjusted net tangible book value gives effect to the (i) issuance of 511,308 shares under of our common stock to Odyssey on December 28, 2023 and (ii) the payment of $1,000,000 in cash to Odyssey.
After giving effect to the sale of our common stock and Pre-funded Warrants in this offering at the public offering price of $ per share and $ per Pre-funded Warrant, and after deducting the underwriting discounts, commissions and estimated offering expenses payable by us and giving effect to i) issuance of 511,308 shares under of our common stock to Odyssey on December 28, 2023 and (ii) the payment of $1,000,000 in cash to Odyssey, our as adjusted, pro forma net tangible book value would be approximately $ , or approximately $ per share of common stock, as of September 30, 2023. This represents an immediate increase in our adjusted, pro forma net tangible book value of approximately $ per share to existing stockholders and an immediate dilution of approximately $ per share to new investors. The following table illustrates this calculation on a per share basis
| Public offering price per share | $ | [___] | ||
| Historical net tangible book value per share as of September 30, 2023 | $ | 6,472,984 | ||
| Adjusted net tangible book value per share as of September 30, 2023 giving effect to the cash paid and common stock issued to Odyssey | $ | 5,472,984 | ||
| As adjusted, pro forma net tangible book value per share after giving effect to this offering | $ | [___] | ||
| Increase in adjusted, pro forma net tangible book value per share attributable to new investors | $ | [___] | ||
| As adjusted, pro forma dilution per share to investors in this offering | $ | [___] |
The adjusted calculation above is based on shares of our common stock outstanding as of September 30, 2023 and excludes as of that date:
| ● | 271,194 shares of our common stock issuable upon the exercise of outstanding options under our equity incentive plans as of January 16, 2024 at a weighted average exercise price of $18.83 per share; | |
| ● | 260,995 shares of common stock reserved for issuance under outstanding warrants as of January 16, 2024 with a weighted average exercise price of $79.04 per share; | |
| ● | 1,004,235 additional shares of common stock reserved for future issuance under our 2021 equity incentive plan as of January 16, 2024; | |
| ● | approximately 9,028 shares of common stock reserved for issuance under conversion of our outstanding shares of Series A Non-Voting, Convertible Preferred Stock; | |
| ● | approximately 13,500 shares of common stock reserved for issuance under conversion of our outstanding shares of Series B Non-Voting, Convertible Preferred Stock; and | |
| ● | approximately 7,488,692 shares of common stock reserved for issuance under conversion of 7,488,692 outstanding shares of Series F Non-Voting, Convertible Preferred Stock. |
The conversion of our outstanding preferred stock may result in further dilution of your investment and, with regard to our our Series F Convertible Preferred Stock, will result in a material further dilution of your investment. Furthermore, to the extent that any outstanding stock options or warrants are exercised, new stock options or warrants are issued, or we otherwise issue additional shares of common stock in the future at a price less than the offering price, there will be further dilution to new investors. See “Risk Factors”.
In addition, we may choose to raise additional capital due to market conditions or strategic considerations, even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
| S-27 |
ThinkEquity LLC, is acting as the representative (the “Representative”) of the underwriters of this offering. We have entered into an underwriting agreement dated , 2024 with the underwriter named below. Pursuant to the terms and subject to the conditions set forth in the underwriting agreement, we have agreed to sell to the underwriter and the underwriter has agreed to purchase, at the public offering price less the underwriting discount set forth on the cover page of this prospectus supplement, the following number of shares of our common stock and Pre-funded Warrants.
| Underwriter | Number of Shares of Common Stock | Number of Pre-funded Warrants |
| ThinkEquity LLC | ||
| Total |
The underwriters are committed to purchase all the shares of common stock and Pre-funded Warrants offered by the Company. The obligations of the underwriter may be terminated upon the occurrence of certain events specified in the underwriting agreement. Furthermore, pursuant to the underwriting agreement, the underwriter’s obligations are subject to customary conditions, representations and warranties contained in the underwriting agreement, such as receipt by the underwriter of officers’ certificates and legal opinions.
We have agreed to indemnify the underwriter against specified liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriter may be required to make in respect thereof.
The underwriter is offering the shares of common stock and Pre-funded Warrants, subject to prior sale, when, as and if issued to and accepted by it, subject to approval of legal matters by its counsel and other conditions specified in the underwriting agreement. The underwriter reserves the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
The underwriter proposes to offer the shares of common stock and Pre-funded Warrants offered by us to the public at the public offering price set forth on the cover of this prospectus supplement. In addition, the underwriter may offer some of the shares of common stock and Pre-funded Warrants to other securities dealers at such price less a concession of $ per share.
Over-Allotment Option. We have granted a 45-day option to the representative of the underwriters to purchase up to ______ shares of our common stock and/or Pre-Funded Warrants solely to cover over-allotments, if any. The underwriters may exercise this option for 45 days from the date of this prospectus supplement solely to cover sales of shares of common stock and Pre-Funded Warrants by the underwriters in excess of the total number of shares of common stock and Pre-Funded Warrants set forth in the table above. If any of these additional shares and Pre-Funded Warrants are purchased, the underwriters will offer the additional shares and Pre-Funded Warrants on the same terms as those on which the shares are being offered.
Discounts and Commissions. The following table shows the public offering price, underwriting discount and proceeds, before expenses, to us. The information assumes either no exercise or full exercise by the underwriters of their over-allotment option.
| Per Share | Per Pre-Funded Warrant | Total Without Over- Allotment Option | Total With Over- Allotment Option | |||||||||||||
| Public offering price | $ | $ | $ | $ | ||||||||||||
| Underwriting discount (7%) | $ | $ | $ | $ | ||||||||||||
| Proceeds, before expenses, to us | $ | $ | $ | $ | ||||||||||||
We have paid an expense deposit of $35,000 (the “Advance”) to the Representative, which will be applied against the actual out-of-pocket accountable expenses that will be paid by us to the underwriters in connection with this offering and will be reimbursed to us to the extent not incurred in compliance with FINRA Rule 5110(g)(4)(A).
We have also agreed to pay certain of the representative’s expenses relating to the offering, including: (a) all fees, expenses and disbursements relating to background checks of our officers, directors and entities in an amount not to exceed $15,000 in the aggregate; (b) fees and expenses of the underwriter’s legal counsel not to exceed $50,000; (c) a $29,500 cost associated with the underwriters use of Ipreo’s book-building, prospectus tracking and compliance software for the offering; (d) $10,000 for data services and communications expenses; (e) up to $30,000 of market making and trading, and clearing firm settlement expenses for the offering; and (f) up to $10,000 of the underwriters’ actual accountable “road show” expenses.
| S-28 |
We have also agreed to pay all expenses relating to the offering, including (a) all filing fees and expenses relating to the registration of the shares of common stock to be sold in the offering with the Commission; (b) all fees associated with the review of the offering by FINRA and all fees and expenses relating to the listing of such shares of common stock on the NYSE American; (c) the costs of all mailing and printing of the underwriting documents; (d) the costs of preparing, printing and delivering certificates representing the common stock sold in this offering; (e) fees and expenses of the Company’s transfer agent; (f) transfer and/or stamp taxes, if any, payable upon the transfer of the shares of our common stock to the underwriter; and (g) fees and expenses of our legal counsel and accountants.
Our total estimated expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding underwriting discounts and commissions and excluding the non-accountable expense allowance, are approximately $ .
Representative’s Warrants. Upon closing of this offering, we have agreed to issue to the representative the Representative’s Warrants to purchase shares of our common stock (5% of the aggregate number of shares of common stock and Pre-Funded Warrants sold in this offering) as a portion of the underwriting compensation payable to the underwriters in connection with this offering. The Representative’s Warrants will be exercisable at a per share exercise price equal to 125% of the public offering price per share in this offering. The Representative’s Warrants are exercisable at any time and from time to time, in whole or in part, during the four and one half year period commencing 180 days from the commencement of sales in this offering.
In addition, the warrants provide for registration rights upon request, in certain cases. The sole demand registration right provided will not be greater than four years beginning on the Initial Exercise Date in compliance with FINRA Rule 5110(g)(8)(C). The piggyback registration rights provided will not be greater than [2] years from the initial exercise date of the Representative’s Warrants in compliance with FINRA Rule 5110(g)(8)(D). We will bear all fees and expenses attendant to registering the securities issuable on exercise of the warrants other than underwriting commissions incurred and payable by the holders. The exercise price and number of shares issuable upon exercise of the warrants may be adjusted in certain circumstances including in the event of a stock dividend or our recapitalization, reorganization, merger or consolidation. However, the warrant exercise price or underlying shares will not be adjusted for issuances of shares of common stock at a price below the warrant exercise price.
Lock-Up Agreements. Pursuant to “lock-up” agreements, we and our executive officers and directors have agreed, for a period of three months from the date of this prospectus supplement, with respect to our officers and directors, and 45 days, with respect to us, not to, directly or indirectly, offer to sell, sell, pledge or otherwise transfer or dispose of any of shares of (or enter into any transaction or device that is designed to, or could be expected to, result in the transfer or disposition by any person at any time in the future of) our common stock, enter into any swap or other derivatives transaction that transfers to another, in whole or in part, any of the economic benefits or risks of ownership of shares of our common stock, make any demand for or exercise any right or cause to be filed a registration statement, including any amendments thereto, with respect to the registration of any shares of common stock or securities convertible into or exercisable or exchangeable for shares of common stock or any other of our securities or publicly disclose the intention to do any of the foregoing, subject to customary exceptions, without the prior written consent of the representative.
Right of First Refusal. We have granted the representative a right of first refusal, for a period of six months from the closing of the offering, to act as sole investment banker, sole book-runner, and/or sole placement agent, at the representative’s sole and exclusive discretion, for each and every future public and private equity and debt offering, including all of our equity linked financings (each, a “Subject Transaction”), or any successor (or any of our subsidiaries), on terms and conditions customary to the representative for such Subject Transactions.
Discretionary Accounts. The underwriter does not intend to confirm sales of the shares of common stock or Pre-funded Warrants offered hereby to any accounts over which it has discretionary authority.
| S-29 |
Electronic Offer, Sale and Distribution of Shares. A prospectus supplement in electronic format may be made available on the websites maintained by the underwriter and the underwriter may distribute prospectus supplements electronically. The underwriter may agree to allocate a number of shares for sale to its online brokerage account holders. Internet distributions will be allocated by the underwriter and on the same basis as other allocations. Other than the prospectus supplement in electronic format, the information on these websites is not part of this prospectus supplement or the registration statement of which this prospectus supplement forms a part, has not been approved or endorsed by us or the underwriter in its capacity as underwriter, and should not be relied upon by investors.
Other Relationships. The underwriter and its affiliates may in the future provide various investment banking, commercial banking and other financial services for us and our affiliates for which it may receive customary fees; however, except as disclosed in this prospectus supplement, we have no present arrangements with the underwriter for any further services.
Price Stabilization, Short Positions and Penalty Bids. In connection with this offering, the underwriter may engage in stabilizing transactions, over-allotment transactions, syndicate covering transactions, penalty bids and purchases to cover positions created by short sales.
| ● | Stabilizing transactions permit bids to purchase shares so long as the stabilizing bids do not exceed a specified maximum and are engaged in for the purpose of preventing or retarding a decline in the market price of our shares while the offering is in progress. | |
| ● | Over-allotment transactions involve sales by the underwriter of shares in excess of the number of shares the underwriter is obligated to purchase. This creates a syndicate short position which may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriter is not greater than the number of shares that it may purchase in the over-allotment option. In a naked short position, the number of shares involved is greater than the number of shares in the over-allotment option. An underwriter may close out any short position by exercising its over-allotment option and/or purchasing shares in the open market. | |
| ● | Syndicate covering transactions involve purchases of shares in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriter will consider, among other things, the price of shares available for purchase in the open market as compared with the price at which it may purchase shares through exercise of the over-allotment option. If the underwriter sells more shares than could be covered by exercise of the over-allotment option and, therefore, has a naked short position, the position can be closed out only by buying shares in the open market. A naked short position is more likely to be created if the underwriter is concerned that after pricing there could be downward pressure on the price of the shares in the open market that could adversely affect investors who purchase in the offering. | |
| ● | Penalty bids permit the underwriter to reclaim a selling concession from a syndicate member when the shares originally sold by that syndicate member are purchased in stabilizing or syndicate covering transactions to cover syndicate short positions. |
These stabilizing transactions, over-allotment transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of shares of our common stock. As a result, the price of our common stock in the open market may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriter make any representation or prediction as to the effect that the transactions described above may have on the price of shares of our common stock. These transactions may be effected on the NYSE American or otherwise.
Passive Market Making. In connection with this offering, the underwriter and selling group members may engage in passive market making transactions in shares of our common stock in accordance with Rule 103 of Regulation M under the Exchange Act during a period before the commencement of offers or sales of the shares and extending through the completion of the distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, that bid must then be lowered when specified purchase limits are exceeded.
| S-30 |
Indemnification. We have agreed to indemnify the underwriters against liabilities relating to this offering arising under the Securities Act and the Exchange Act, liabilities arising from breaches of some, or all of the representations and warranties contained in the underwriting agreement, and to contribute to payments that the underwriters may be required to make for these liabilities.
Offer restrictions outside the United States
Other than in the United States, no action has been taken by us or the underwriter that would permit a public offering of the securities offered by this prospectus supplement in any jurisdiction where action for that purpose is required. The securities offered by this prospectus supplement may not be offered or sold, directly or indirectly, nor may this prospectus supplement or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons who receive this prospectus supplement are advised to inform themselves about, and to observe, any restrictions relating to this offering and the distribution of this prospectus supplement. This prospectus supplement does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus supplement in any jurisdiction in which such an offer or a solicitation is unlawful.
The validity of the issuance of the securities offered hereby has been passed upon for us by Shumaker, Loop & Kendrick, LLP. Sichenzia Ross Ference Carmel LLP, New York, New York, has acted as counsel for the underwriter.
The consolidated financial statements of Oragenics, Inc. (“Company”) as of and for the years ended December 31, 2022 and 2021, appearing in the Company’s Annual Report on Form 10-K for the year ended December 31, 2022, have been audited by Mayer Hoffman McCann P.C., independent registered public accounting firm, as set forth in their report (which report includes an explanatory paragraph regarding the existence of substantial doubt about the Company’s ability to continue as a going concern), and have been incorporated herein by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing, in giving said reports.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-3 (File No. 333-269225) under the Securities Act of 1933, as amended, or the Securities Act, with respect to the securities offered by this prospectus supplement and the accompanying prospectus. This prospectus supplement and the accompanying prospectus filed as part of the registration statement do not contain all the information set forth in the registration statement and its exhibits. For further information about us, we refer you to the registration statement and to its exhibits.
We are a public company and file annual, quarterly and current reports, proxy statements and other information with the Securities and Exchange Commission (“SEC”).You can request copies of these documents by writing to the SEC and paying a fee for the copying cost. Our SEC filings are also available to the public at the SEC’s web site at http://www.sec.gov.
In addition, we maintain a web site that contains information regarding our company, including copies of reports, proxy statements and other information we file with the SEC. The address of our web site is www.oragenics.com. Except for the documents specifically incorporated by reference into this prospectus, information contained on our website or that can be accessed through our website does not constitute a part of this prospectus. We have included our website address only as an inactive textual reference and do not intend it to be an active link to our website.
| S-31 |
INFORMATION INCORPORATED BY REFERENCE
In this document, we “incorporate by reference” certain information we file with the SEC, which means that we can disclose important information to you by referring to that information. The information incorporated by reference is considered to be a part of this prospectus supplement. Any statement contained in a document incorporated by reference herein shall be deemed to be modified or superseded for all purposes to the extent that a statement contained in this prospectus supplement or in any other subsequently filed document that is also incorporated or deemed to be incorporated by reference, modifies or supersedes such statement. Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus supplement. We incorporate by reference the documents listed below (other than, in each case, documents or information deemed to be furnished and not filed in accordance with SEC rules):
| ● | Our Annual Report on Form 10-K for the year ended December 31, 2022, filed with the SEC on April 17, 2023; |
| ● | Our Quarterly Reports on Form 10-Q for the quarter ended March 31, 2023, filed with the SEC on May 12, 2023, for the quarter ended June 30, 2023 filed with the SEC on August 11, 2023 and for the quarter ended September 30, 2023 filed with the SEC on November 9, 2023; |
| ● | Our Definitive Proxy Statement on Schedule 14A, filed with the SEC on October 30, 2023; |
| ● | Our Current Reports on Form 8-K, filed January 23, 2023, February 3, 2023, February 24, 2023, March 1, 2023, March 8, 2023, March 23, 2023, April 10, 2023, May 12, 2023, August 7, 2023, August 10, 2023, August 18, 2023, September 19, 2023, September 29, 2023, October 5, 2023, October 5, 2023, November 2, 2023, November 20, 2023, December 8, 2023, December 15, 2023, December 29, 2023 and January 2, 2024; and |
| ● | The description of our common stock set forth in our registration statement on Form 8-A12B, filed April 8, 2013, including any amendments or reports filed for purposes of updating such description. |
We also incorporate by reference into this prospectus supplement and the accompanying prospectus all documents (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items) that are filed by us with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, after the date of this prospectus supplement until we sell all of the securities covered by this prospectus supplement and the accompanying prospectus or the sale of securities by us pursuant to this prospectus supplement and the accompanying prospectus is terminated.
A statement contained in a document incorporated by reference into this prospectus supplement and the accompanying prospectus shall be deemed to be modified or superseded for purposes of this prospectus supplement and the accompanying prospectus to the extent that a statement contained in this prospectus supplement and the accompanying prospectus or in any other subsequently filed document which is also incorporated by reference in this prospectus supplement and the accompanying prospectus modifies or replaces such statement. Any statements so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus supplement and the accompanying prospectus.
We hereby undertake to provide without charge to each person, including any beneficial owner, to whom a copy of this prospectus supplement is delivered, upon written or oral request of any such person, a copy of any and all of the information that has been or may be incorporated by reference in this prospectus supplement, including any exhibits that are specifically incorporated by reference in such documents. Requests for such copies should be directed as follows: Oragenics, Inc., 4902 Eisenhower Boulevard, Suite 125, Tampa, Florida 33634, Attention: Investor Relations, Phone: (813) 276-7900.
| S-32 |
PROSPECTUS

$40,000,000
Common Stock
Warrants
Units
From time to time, we may offer, issue and sell up to $40,000,000 of any combination of the securities described in this prospectus. We may also offer securities as may be issuable upon conversion, redemption, repurchase, exchange or exercise of any securities registered hereunder, including any applicable antidilution provisions.
This prospectus provides you with a general description of the securities we may offer. Each time we offer securities, we will provide the specific terms of these offerings and securities in one or more supplements to this prospectus. We may also authorize one or more free writing prospectuses to be provided to you in connection with these offerings. The prospectus supplement and any related free writing prospectus may also add, update or change information contained in this prospectus. You should carefully read this prospectus, the applicable prospectus supplement and any related free writing prospectus, as well as any documents incorporated by reference, before buying any of the securities being offered.
This prospectus may not be used to consummate a sale of any securities unless accompanied by a prospectus supplement.
The securities may be sold directly by us to investors, through agents designated from time to time or to or through underwriters or dealers, on a continuous or delayed basis. For additional information on the methods of sale, you should refer to the section entitled “Plan of Distribution” in this prospectus and in the applicable prospectus supplement. If any agents or underwriters are involved in the sale of any securities with respect to which this prospectus is being delivered, the names of such agents or underwriters and any applicable fees, commissions, discounts and over-allotment options will be set forth in a prospectus supplement. The price to the public of such securities and the net proceeds that we expect to receive from such sale will also be set forth in a prospectus supplement.
Our common stock is listed on the NYSE American under the symbol “OGEN”. The last reported sale price of our common stock on January 12, 2023 was $7.63 per share. The applicable prospectus supplement will contain information, where applicable, as to any other listing, if any, on the NYSE American or any securities market or other exchange of the securities covered by the applicable prospectus supplement.
As of January 12, 2023, the aggregate market value of our outstanding common stock held by non-affiliates, or the public float, was approximately $14,662,868, which was calculated based on 1,921,739 shares of our outstanding common stock held by non-affiliates and on a price of $7.63 per share, the last reported sale price for our common stock on January 12, 2023. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell our securities in a public primary offering with a value exceeding one-third of our public float in any 12-month period unless our public float subsequently rises to $75.0 million or more.
Investing in our securities involves a high degree of risk. You should review carefully the risks and uncertainties described under the heading “Risk Factors” beginning on page 8 of this prospectus, or contained in the applicable prospectus supplement and any related free writing prospectus we have authorized for use in connection with a specific offering, and under similar headings in the other documents that are incorporated by reference into this prospectus.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is January 25, 2023.
TABLE OF CONTENTS
This prospectus is part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission, or SEC, utilizing a “shelf” registration process. Under this shelf registration statement, we may, from time to time, sell any combination of the securities referred to herein in one or more offerings for total gross proceeds of up to $40,000,000. This prospectus provides you with a general description of the securities we may offer.
Until such time, if ever, as we are eligible to use General Instruction I.B.1. of Form S-3, pursuant to General Instruction I.B.6. of Form S-3, we are permitted to use the registration statement of which this prospectus forms a part to sell, via a primary offering, a maximum amount of securities equal to one-third of the aggregate market value of our outstanding voting and non-voting common equity held by non-affiliates of our company in any twelve month period.
Each time we offer a type or series of securities under this prospectus, we will provide a prospectus supplement that will contain more specific information about the terms of the offered securities. We also may authorize one or more free writing prospectuses to be provided to you that may contain material information relating to these offerings. This prospectus, together with applicable prospectus supplements and any related free writing prospectuses, includes all material information relating to these offerings. We also may add, update or change, in the prospectus supplement and in any related free writing prospectus that we may authorize to be provided to you, any of the information contained in this prospectus or in the documents that we have incorporated by reference into this prospectus. We urge you to read carefully this prospectus, any applicable prospectus supplement and any related free writing prospectus, together with the information incorporated herein by reference as described under the section entitled “Where You Can Find Additional Information” and “Incorporation of Certain Information by Reference” in this prospectus, before investing in any of the securities offered.
THIS PROSPECTUS MAY NOT BE USED TO CONSUMMATE A SALE OF SECURITIES UNLESS IT IS ACCOMPANIED BY A PROSPECTUS SUPPLEMENT.
You should rely only on the information that we have provided or incorporated by reference in this prospectus, any applicable prospectus supplement and any related free writing prospectus that we may authorize to be provided to you. We have not authorized any other person to provide you with different or additional information. No dealer, salesperson or other person is authorized to give any information or to represent anything not contained in this prospectus, any applicable prospectus supplement or any related free writing prospectus that we may authorize to be provided to you. You must not rely on any unauthorized information or representation. This prospectus, any applicable supplement to this prospectus or any related free writing prospectus do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the registered securities to which they relate, nor do this prospectus, any applicable supplement to this prospectus or any related free writing prospectus constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction.
You should not assume that the information appearing in this prospectus, any applicable prospectus supplement or any related free writing prospectus is accurate on any date subsequent to the date on the front of the document and that any information we have incorporated by reference is accurate as of the date of the document incorporated by reference, but not on any date subsequent to the date of the document incorporated by reference, regardless of the time of delivery of this prospectus, any applicable prospectus supplement or any related free writing prospectus or any sale of a security. Our business, financial condition, results of operations and prospectus may have changed since those dates.
This prospectus contains and incorporates by reference market data, industry statistics and other data that have been obtained from, or compiled from, information made available by third parties. We have not independently verified their data. This prospectus and the information incorporated herein by reference include trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included or incorporated by reference into this prospectus, any applicable prospectus supplement or any related free writing prospectus are the property of their respective owners.
This prospectus and the information incorporated herein by reference contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed, or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the section entitled “Where You Can Find Additional Information” and “Incorporation of Certain Information by Reference”.
| 1 |
The items in the following summary are described in more detail elsewhere in this prospectus and in the documents incorporated by reference herein. This summary provides an overview of selected information and does not contain all the information you should consider before investing in our common stock. Therefore, you should read the entire prospectus and any free writing prospectus that we have authorized for use in connection with this offering carefully, including the “Risk Factors,” and information under similar headings in the other documents that are incorporated by reference into this prospectus. You should also carefully read the information incorporated by reference into this prospectus, including our financial statements and related notes, and the exhibits to the registration statement of which this prospectus is a part, before making any investment decision.
Unless otherwise mentioned or unless the context requires otherwise, all references in this prospectus to “Oragenics” the “Company,” “we,” “our” and “us” or similar references mean Oragenics, Inc. When we refer to “you,” we mean the holders of the applicable securities. We own various U.S. federal trademark applications and unregistered trademarks, including our company name. All other trademarks or trade names referred to in this prospectus are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the symbols ® and ™, but such references should not be construed as any indication that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.
Overview
We are a development-stage company dedicated to fighting infectious diseases including coronaviruses and multidrug-resistant organisms. Our lead product (NT-CoV2-1) is an intranasal vaccine candidate to prevent coronavirus disease (“COVID-19”) from the SARS-CoV-2 virus and variants thereof. The NT-CoV2-1 program leverages coronavirus spike protein research licensed from the National Institutes of Health and the National Research Council of Canada with a focus on reducing viral transmission and offering a more patient-friendly intranasal administration. Our lantibiotics program features a novel class of antibiotics against bacteria that have developed resistance to commercial antibiotics.
Our SARS-CoV-2 Vaccine Product Candidate - NT-CoV2-1
Following our May 2020 acquisition of one hundred percent (100%) of the total issued and outstanding common stock of Noachis Terra, Inc. (“Noachis Terra”) we are focused on the development and commercialization of a vaccine product candidate to provide long-lasting immunity from SARS-CoV-2, which causes COVID-19. Noachis Terra is a party to a worldwide, nonexclusive intellectual property and biological materials license agreement with the National Institute of Allergy and Infectious Diseases (“NIAID”), an institute within the National Institutes of Health (“NIH”), relating to certain research, patent applications and biological materials involving pre-fusion stabilized coronavirus spike proteins and their use in the development and commercialization of a vaccine to provide specific, long lasting immunity from SARS-CoV-2. Since the acquisition we have conducted testing in animal models, including SARS-CoV-2 challenge studies in hamsters, using specific formulations for intramuscular administration (our Terra CoV-2 vaccine candidate) and intranasal administration (our NT-CoV2-1 vaccine candidate), both based on the NIAID pre-fusion stabilized spike protein antigens. Following consideration of a number of factors, including but not limited to the competitive landscape, we determined to bring the intranasal vaccine candidate NT-CoV2-1, into further development due to the greater differentiation versus current COVID-19 vaccines and the potential benefits of intranasal over intramuscular administration. We believe these benefits could include a higher reduction of transmission of SARS-CoV-2 and would offer a needle-free delivery option. We therefore are currently focusing our development efforts on our more highly differentiated NT-CoV2-1 vaccine candidate.
On July 26, 2021, we entered into a licensing agreement with the National Research Council (“NRC”) that enables us to pursue the development of next-generation vaccines against the SARS-CoV-2 virus and its variants. The license was subsequently amended to: include the Omicron variant, broaden the non-exclusive field of use to include all diseases caused by coronaviruses, and any genetic variants thereof, to add a research protocol developed by the NRC, and to add reagents as part of the NRC Technology licensed by us. The NRC technologies, in combination with the licensed technologies from the U.S. NIH used in our NT-CoV2-1 vaccine candidate, provide us with a platform that can generate cell lines for high-yield production of spike protein antigens for existing and emerging variants of concern. This platform should allow production of cell lines within six to eight weeks of spike gene sequence availability, compared with six to nine months for traditional production of such cell lines. The NRC technologies, developed with support from the NRC’s Pandemic Response Challenge Program, are expected to enable expedited evaluation of SARS-CoV-2 antigen candidates in pre-clinical and clinical studies.
| 2 |
Coronaviruses are a family of viruses that can lead to upper-respiratory infections in humans. Recent clinical reports also suggest that the SARS-CoV-2 virus can affect other body-systems, including the nervous, cardiovascular, gastrointestinal and renal systems. Among the recent iterations of coronaviruses to move from animal to human carriers is SARS-CoV-2, which, beginning in Wuhan, China, in late 2019, caused a global pandemic due to its rapid spread and the relatively high mortality rate (as compared to the seasonal influenza). Pfizer/BioNTech received FDA approval for their COVID-19 vaccines in August of 2021 and the Moderna vaccine in January 2022. The Janssen vaccine is currently available in the United States under Emergency Use Authorizations (“EUA”) by the FDA. In July 2022, the FDA granted EUA for the Novavax COVID-19 vaccine as well. Current vaccines have reduced the rates of hospitalization and death due to COVID-19 in vaccinated individuals, but the transmission levels even in vaccinated individuals has allowed SARS-CoV-2 variants to continue to circulate. We believe given the size of the worldwide spread of COVID-19 that even with additional vaccines available, there will be demand for the highly differentiated NT-CoV2-1 vaccine, once development is successfully completed. We intend to combine the research, patent applications and biological materials covered by our NIAID license and with our NRC license and our existing clinical research and manufacturing capabilities to respond to this ongoing, global, public health issue. We believe our NT-CoV2-1 vaccine holds the possibility of playing an important role in addressing this issue.
Coronaviruses, such as SARS-CoV-2, possess signature protein spikes on their outer capsule. Our NIAID license covers patents and data on a vaccine candidate that were created based on a stabilized pre-fusion spike trimeric protein. By stabilizing the spike protein in the pre-fusion state, the number of immunogenic centers is increased thereby allowing for a greater likelihood of successful antibody binding, resulting in an improved immunogenic response. Spike protein antigens stabilized in the pre-fusion state have been used successfully in the leading COVID-19 vaccines from Pfizer/BioNTech and Moderna, which we believe reduces the risk of using the same approach in our NT-CoV2-1 vaccine candidate. The genetic code, acquired from the NIH, for the stabilized pre-fusion spike protein was provided to Aragen Bioscience, Inc. (“Aragen”) for the purpose of insertion of the spike protein gene sequence into a Chinese Hamster Ovary (“CHO”) cell line. Aragen is a leading contract research organization focused on accelerating pre-clinical biologics product development, has extensive experience building CHO cell lines for recombinant proteins, such as monoclonal antibodies. Aragen successfully inserted the NIH pre-fusion spike protein gene sequence into a CHO cell line and Oragenics is currently producing Phase 1 clinical material based upon this cell line.
We entered into both a material transfer agreement and a non-exclusive research license agreement with Inspirevax for the use of intranasal mucosal adjuvants in our NT-CoV2-1 vaccine candidates. Regarding the intranasal mucosal adjuvants of interest, BDX300 and BDX301 are proteosome-based adjuvants comprised of proteins and lipopolysaccharides with improved attributes including enhanced immune response, manufacturing efficiency and the benefits of intranasal vaccine administration. The non-exclusive license agreement allows for the collaboration and research regarding the intranasal delivery of vaccine during clinical development with the opportunity to enter into a commercial agreement upon regulatory approval of the intranasal vaccine. The NT-CoV2-1 vaccine containing Inspirevax’s intranasal mucosal adjuvant BDX301 has been studied in pre-clinical animal studies, including hamster viral challenge studies and mouse immunogenicity studies. A rabbit toxicology study has been initiated and is required for regulatory approval prior to the Phase 1 clinical study.
A Non-Exclusive Research License Agreement with Inspirevax was executed in February 2022. This agreement granted the Company non-exclusive rights to conduct non-clinical and clinical research and trials in relation to vaccines comprising the BDX300 or BDX301 adjuvants to prevent or treat diseases caused by coronaviruses and genetic variants thereof.
We began pre-clinical studies in June of 2021 through our collaboration and material transfer agreement with the NRC. We initiated an immunogenicity study in mice to evaluate several adjuvant candidates. On August 30, 2021, we announced the successful completion of these mouse immunogenicity studies that supported further development using either the intramuscular or intranasal routes of administration. A hamster challenge study was initiated in September of 2021 to assess inhibition of viral replication using adjuvants specific for intramuscular and intranasal administration. In December of 2021, we announced that both formulations generated robust immune responses and reduced the SARS-CoV-2 viral loads to undetectable levels in the nasal passages and lungs five days following a viral challenge. By contrast, hamsters in the control groups that had received saline or adjuvants alone had no detectable immune response and substantial viral loads. The vaccines delivered by intranasal and intramuscular routes generated immune responses as measured by multiple assays. On June 14, 2022, we announced that the results of these studies were published in Nature Scientific Reports.
| 3 |
In March of 2022, following a positive assessment of a rabbit-based pilot study, we initiated a Good Laboratory Practice toxicology study to evaluate the safety profile and immunogenicity of NT-CoV2-1 in rabbits. This important preclinical study is designed to provide data required to advance our intranasal vaccine candidate into human clinical studies. The study has concluded and we completed the full set of toxicology data, which is needed to support the filing of an IND application for NT-CoV2-1. Based on the findings of the final toxicology report, including a full histopathology evaluation, we were able to confirm a safety and immunogenicity profile that further support our plan to submit regulatory filings required to progress to a Phase 1 clinical study.
While we previously had a Type B Pre-IND Meeting with the FDA on our intramuscular vaccine product candidate, we again met with the FDA in a Type B Pre-IND Meeting request to discuss our intranasal vaccine product candidate. As a result of this meeting, the FDA indicated that the Company could file an IND application for NT-CoV2-1 following the availability of the final GLP toxicology report for inclusion in the IND.
We believe the benefits of our NT-CoV2-1 vaccine product candidate through its intranasal delivery mechanism to be:
| ● | Targeted Mucosal Immunity – Conventional injectable vaccines are poor inducers of mucosal immunity, whereas intranasal immunization can induce strong mucosal immunity by enhancing the immune response at the entry sites of mucosal pathogens. When the SARS-CoV-2 virus enters the nasal cavity, the respiratory epithelial layer is the first barrier against viral infection. The intranasal route of vaccination provides two additional layers of protection over intramuscular shots because (i) it produces immunoglobulin A and resident memory B and T cells in the respiratory mucosa that are an effective barrier to infection at those sites, and (ii) cross-reactive resident memory B and T cells can respond earlier than other immune cells should a viral variant start an infection. |
| ● | Needle-Free Administration – As an obvious benefit, intranasal administration means needle-free delivery, resulting in meaningful differentiation for children and needle-phobic populations, improved compliance and the potential for self-administration. |
| ● | Storage & Transport – The currently available mRNA-based vaccines have been delivered globally via stringent storage and transport requirements that strain distribution logistics under the best of circumstances. A key benefit of our NT-CoV2-1 vaccine candidate is a significantly reduced handling burden, allowing transport at a more manageable refrigeration temperature (5°C) that improves access globally including remote and under-vaccinated geographies. |
| ● | Durability – Broad initial success with mRNA vaccines has significantly diminished COVID-19’s impact and death, but the trade-off has been fleeting efficacy. By benefitting from the immunological properties of the hybrid NIH/NRC construct, NT-CoV2-1 is potentially much more durable and long-lasting than currently available mRNA-based therapies. |
Through assessment of a variety of factors including our pre-clinical testing to date, the expected benefits noted above, evolving variants and available vaccines in use, we determined to focus our development efforts on the intranasal delivery of our vaccine product candidate, NT-CoV2-1, which we believe is more highly differentiated than the currently available and late-stage COVID-19 vaccines. We expect to seek to file an IND application with the FDA and to thereafter commence a Phase 1 clinical study with NT-CoV2-1, the protocol for which is currently under development.
| 4 |
We expect to use our currently available cash resources to continue to advance the development of NT-CoV2-1 through IND-enabling studies and commencement of a Phase 1 clinical trial with further clinical development being contingent upon the receipt of additional funding, including non-dilutive government grant funding which we continue to pursue, or partnering or out-licensing opportunities.
Our Antibiotic Product Candidate - Oragenics Derived Compound (ODC-x)
Members of our scientific team discovered that a certain bacterial strain of Streptococcus mutans, produces Mutacin 1140 (MU1140), a molecule belonging to the novel class of antibiotics known as lantibiotics. Lantibiotics, such as MU1140, are highly modified peptide antibiotics made by a small group of Gram-positive bacterial species. Over 60 lantibiotics have been discovered, to date. We believe lantibiotics are generally recognized by the scientific community to be potent antibiotic agents.
In nonclinical testing, MU1140 has shown activity against all Gram-positive bacteria against which it has been tested, including those responsible for a number of healthcare associated infections, or HAIs. A high percentage of hospital-acquired infections are caused by highly antibiotic-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) or multidrug-resistant Gram-negative bacteria. We believe the need for novel antibiotics is increasing as a result of the growing resistance of target pathogens to existing FDA approved antibiotics on the market.
Lantibiotics have been difficult to investigate for their clinical usefulness as therapeutic agents in the treatment of infectious diseases due to a general inability to produce or synthesize sufficient quantities of pure amounts of these molecules. Traditional fermentation methods can only produce minute amounts of the lantibiotic.
The timing of the filing of an IND regarding any future lantibiotic candidate is subject to our having sufficient available human, material and financing capital, which includes research subjects, both animal and human, given all of our anticipated needs and expected requirements in connection with our ongoing research and development initiatives. We expect to continue to advance our lantibiotics program to an IND filing based on the availability of both human and financial capital. Based upon the current funding we expect to continue to focus on the identification of new potential product lantibiotic candidates, efficient and cost-effective improvements in the manufacturing processes and pre-clinical studies required to support a first in human Phase 1 clinical study.
In October 2021, we were awarded a small business innovation research grant in the amount of $250,000 (“Computer-aided Design for Improved Lantibiotics”, R41GM136034) for the Company’s continued research and development of lantibiotics, including its collaborative program with the Biomolecular Sciences Institute at Florida International University (FIU). The grant provides the Company with funding to develop novel lantibiotics for the treatment of ESKAPE pathogens (defined as Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.).
Product Candidates.
Through our wholly-owned subsidiary, Noachis Terra, we began the research and development stage for our new Terra CoV-2 and NT-CoV2-1 vaccine product candidates. We hold a nonexclusive, worldwide intellectual property license agreement for certain research, patent applications and biological materials relating to the use of pre-fusion coronavirus spike proteins for the development and commercialization of a vaccine against SARS-CoV-2. We also hold a non-exclusive license with the NRC that enables us to pursue the rapid development of next-generation vaccines against the SARS-CoV-2 (the “NIH License”) virus and its variants (the “NRC License” and together with the NIH License the “License Agreements”).
Additionally, we are developing semi-synthetic lantibiotic analogs that may be effective against systemic Gram-positive multidrug infections, and analogs that may be effective in treating Gram-negative infections. We seek to protect our product candidates through patents and patent applications pursuant to the terms of our License Agreements.
| 5 |
| Product/Candidate | Description | Application | Status | |||
| NT-CoV2-1 | Intranasal vaccine candidate (recombinant protein + adjuvant) to provide long lasting immunity against SARS-CoV-2 | Broad, community-based vaccine immunity against SARS-CoV-2 | Pre-clinical | |||
| Antibiotics | Semi-synthetic analogs of MU1140: Member of lantibiotic class of antibiotics | Healthcare-associated infections | Pre-clinical |
Recent Developments
On December 22, 2022 our board of directors approved a 1 for 60 reverse stock split of our authorized, issued and outstanding of common stock to be effective on January 20, 2023. The par value per common shares will remain unchanged. Except where the context otherwise requires, share numbers in this prospectus reflect the 1 for 60 reverse stock split of our common stock.
Our Business Development Strategy
Success in the biopharmaceutical and product development industry relies on the continuous development of novel product candidates. Most product candidates do not make it past the clinical development stage, which forces companies to look externally for innovation. Accordingly, we expect from time to time, to seek strategic opportunities through various forms of business development, which can include strategic alliances, licensing deals, joint ventures, collaborations, equity-or debt-based investments, dispositions, mergers and acquisitions. We view these business development activities as a necessary component of our strategies, and we seek to enhance shareholder value by evaluating business development opportunities both within and complementary to our current business as well as opportunities that may be new and separate from the development of our existing product candidates.
Corporate and Other Information
We were incorporated in November 1996 and commenced operations in 1999. We consummated our initial public offering in June 2003. Our executive office is located at, 4902 Eisenhower Boulevard, Suite 125 Tampa, Florida, 33634 and our research facilities are located at 13700 Progress Boulevard, Alachua, Florida 32615. Our telephone number is (813) 286-7900 and our website is http://www.oragenics.com. We make available free of charge on our website our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports as soon as reasonably practicable after we electronically file or furnish such materials to the Securities and Exchange Commission (the “SEC”). The reports are also available at www.sec.gov. We do not incorporate by reference into this prospectus the information on, or accessible through, our website, and you should not consider it as part of this prospectus and it should not be relied on in connection with this offering. We have included our website address as an inactive textual reference only.
Implications of Being a Smaller Reporting Company
We are a “smaller reporting company” as defined in Rule 12b-2 promulgated under the Securities Exchange Act of 1934, as amended, or the Exchange Act. We may remain a smaller reporting company until we have a non-affiliate public float in excess of $250 million and annual revenues in excess of $100 million, or a non-affiliate public float in excess of $700 million, each as determined on an annual basis. A smaller reporting company may take advantage of relief from some of the reporting requirements and other burdens that are otherwise applicable generally to public companies. These provisions include:
| ● | being permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure; | |
| ● | not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting; and | |
| ● | reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements. |
| 6 |
Securities We May Offer
We may offer shares of our common stock, warrant shares of our common stock to purchase, either individually or in combination, and/or units consisting of some or all of such securities for total gross proceeds of up to $40 million, from time to time under this prospectus, together with the applicable prospectus supplement and any related free writing prospectus, at prices and on terms to be determined by market conditions at the time of any offering. This prospectus provides you with a general description of the securities we may offer. We will describe in the applicable prospectus supplement relating to any securities the particular terms of the securities offered by that prospectus supplement. If we indicate in the applicable prospectus supplement, the terms of the securities may differ from the terms we have summarized below. We may also include in the prospectus supplement information about material United States federal income tax considerations relating to the securities, and the securities exchange, if any, on which the securities will be listed.
We may sell from time to time, in one or more offerings:
| ● | Common stock; | |
| ● | Warrants to purchase shares of common stock; and | |
| ● | Units consisting of any combination of the securities listed above. |
In this prospectus, we refer to the common stock, warrants and units collectively as “securities”. The total dollar amount of all securities that we may sell pursuant to this prospectus will not exceed $40,000,000.
This prospectus may not be used to consummate a sale of securities unless it is accompanied by a prospectus supplement.
| 7 |
Investing in our securities involves a high degree of risk. You should carefully review the risks and uncertainties described under the heading “Risk Factors” contained in the applicable prospectus supplement and any related free writing prospectus, and under similar headings in our Annual Report on Form 10-K for the year ended December 31, 2021, as updated or supplemented by any subsequently filed periodic reports and other documents as filed with the SEC and incorporated by reference into this prospectus, before deciding whether to purchase any of the securities being registered pursuant to the registration statement of which this prospectus is a part. Each of the risk factors described in the documents referenced above could adversely affect our business, operating results and financial condition, as well as adversely affect the value of an investment in our securities, and the occurrence of any of these risks might cause you to lose all or part of your investment. Additional risks not presently known to us or that we currently believe are immaterial may also significantly impair our business operations.
| 8 |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference herein contain forward-looking statements. These are based on our management’s current beliefs, expectations and assumptions about future events, conditions and results and on information currently available to us. Discussions containing these forward-looking statements may be found, among other places, in the sections entitled “Business,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained in the documents incorporated by reference herein.
Any statements in this prospectus, or incorporated herein, about our expectations, beliefs, plans, objectives, assumptions or future events or performance are not historical facts and are forward-looking statements. Within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act, these forward-looking statements include statements regarding:
| ● | We have incurred significant operating losses since our inception and cannot assure you that we will generate revenues or achieve profitability; | |
| ● | We will need to raise additional capital to fully implement our business strategy and we may not be able to do so; | |
| ● | Our financial capacity and performance, including our ability to obtain funding, non-dilutive or otherwise, necessary to do the research, development, manufacture and commercialization of any one or all of our product candidates; | |
| ● | The timing, progress and results of clinical trials of our product candidates, including statements regarding the timing of initiation and completion of pre-clinical studies or clinical trials or related preparatory work, the period during which the results of the trials will become available and our research and development programs; | |
| ● | The timing of any submission of filings for regulatory approval of our product candidates and our ability to obtain and maintain regulatory approvals for our product candidates for any indication; | |
| ● | Our expectations regarding the potential benefits, activity, effectiveness and safety of our product candidates including as to administration, distribution and storage; | |
| ● | Our expectations regarding the size of the patient populations, market acceptance and opportunity for and clinical utility of our product candidates, if approved for commercial use; | |
| ● | Our manufacturing capabilities and strategy, including the scalability and commercial viability of our manufacturing methods and processes, and those of our contractual partners; | |
| ● | Our expectations regarding the scope of any approved indications for our product candidates; | |
| ● | Our ability to successfully commercialize our product candidates; | |
| ● | The potential benefits of, and our ability to maintain, our relationships and collaborations with the NIAID, the NIH, the NRC and other potential collaboration or strategic relationships; | |
| ● | Our ability to use our lantibiotic platform to develop future product candidates; |
| ● | Our estimates of our expenses, ongoing losses, future revenue, capital requirements and our needs for or ability to obtain additional funding, including any application for future grants or funding; | |
| ● | Our ability to identify, recruit and retain key personnel and consultants; |
| 9 |
| ● | Our ability to obtain, retain, protect and enforce our intellectual property position for our product candidates, and the scope of such protection; | |
| ● | Our ability to advance the development of our new NT-CoV2-1 vaccine product candidate under the timelines and in accord with the milestones projected; | |
| ● | Our inability to achieve success in our identification of lantibiotic homologs or the manufacture and nonclinical testing of our lantibiotic product candidates; | |
| ● | Our need to comply with extensive and costly regulation by worldwide health authorities, who must approve our product candidates prior to substantial research and development and could restrict or delay the future commercialization of certain of our product candidates; | |
| ● | Our ability to successfully complete pre-clinical and clinical development of, and obtain regulatory approval of our product candidates and commercialize any approved products on our expected timeframes or at all; | |
| ● | The safety, efficacy and benefits of our product candidates; | |
| ● | The content and timing of submissions to and decisions made by the FDA, other regulatory agencies and nongovernmental bodies and actors, such as investigational review boards; | |
| ● | The effects of government regulation and regulatory developments, and our ability and the ability of the third parties with whom we engage to comply with applicable regulatory requirements; | |
| ● | The capacities and performance of our suppliers and manufacturers and other third parties over whom we have limited control; | |
| ● | Our ability to maintain our listing on the NYSE American and the effects of our contemplated 1 for 60 reverse stock split on our price per share and the trading market of our common stock; | |
| ● | The impact of the COVID-19 pandemic on our financial condition and business operations and our ability to continue research and development for existing product candidates on previously-projected timelines or in accord with ordinary practices, as well as the broader governmental, global health and macro- and microeconomic responses to and consequences of the pandemic; | |
| ● | We may be adversely impacted by any significant broad-based financial crises and its impact on consumers, retailers and equity and debt markets as well as our inability to obtain required additional funding to conduct our business; | |
| ● | As a public company, we must implement additional and expensive finance and accounting systems, procedures and controls as we grow our business and organization to satisfy reporting requirements, which add to our costs and require additional management time and resources; | |
| ● | Our competitive position and the development of and projections relating to our competitors or our industry; and | |
| ● | The impact of laws and regulations, including those that may not yet exist. |
In some cases, you can identify forward-looking statements by the words “may,” “might,” “can,” “will,” “to be,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “likely,” “continue” and “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future, although not all forward-looking statements contain these words. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements.
You should refer to the “Risk Factors” section contained in the applicable prospectus supplement and any related free writing prospectus, and under similar headings in the other documents that are incorporated by reference into this prospectus, for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. Given these risks, uncertainties and other factors, many of which are beyond our control, we cannot assure you that the forward-looking statements in this prospectus will prove to be accurate, and you should not place undue reliance on these forward-looking statements. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all.
Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to revise any forward-looking statements to reflect events or developments occurring after the date of this prospectus, even if new information becomes available in the future.
| 10 |
We will retain broad discretion over the use of the net proceeds from the sale of the securities offered hereby. Except as described in any applicable prospectus supplement or in any free writing prospectuses that we may authorize to be provided to you in connection with a specific offering, we currently intend to use the net proceeds from the sale of the securities offered hereby for working capital, capital expenditures and general corporate purposes, which may include, without limitation, funding research, clinical and process development and manufacturing of our product candidates. We may also use a portion of the net proceeds to invest in, collaborate with, acquire, or in-licensing of products or product candidates, business or technologies that we believe are complementary to our own, although we have no current plans, commitments or agreements with respect to any acquisitions as of the date of this prospectus. We will set forth in the applicable prospectus supplement or free writing prospectus our intended use for the net proceeds received from the sale of any securities sold pursuant to the prospectus supplement or free writing prospectus. Pending these uses, we intend to invest the net proceeds in investment-grade, interest-bearing securities.
| 11 |
We have never paid cash dividends on our common stock. Moreover, we do not anticipate paying periodic cash dividends on our common stock for the foreseeable future. We intend to use all available cash and liquid assets in the operation and growth of our business. Any future determination about the payment of dividends will be made at the discretion of our board of directors and will depend upon our earnings, if any, capital requirements, operating and financial conditions and on such other factors as our board of directors deems relevant.
| 12 |
The following descriptions are summaries of the material terms that are included in our amended and restated articles of incorporation (as amended) and our bylaws (as amended) as well as the specific agreements such descriptions relate to. This summary is qualified in its entirety by the specific terms and provisions contained in our restated articles of incorporation, bylaws and the specific agreements described herein, copies of which we have filed as exhibits to the registration statement of which this prospectus is a part, and by the provisions of applicable law.
Overview
Authorized Capital Stock
Our authorized capital stock consists of 250,000,000 (4,166,666 following the effectiveness of our 1 for 60 reverse stock on January 20, 2023) shares of common stock, par value $0.001, and 50,000,000 shares of preferred stock, without par value.
Common Stock
Voting
The holders of our common stock are entitled to one vote for each share held of record on all matters submitted to a vote of the shareholders. Approval of an amendment of our articles of incorporation, a merger, a share exchange, a sale of all our property or dissolution must be approved by a majority of all votes entitled to be cast. Such votes may be cast in person or by proxy as provided in Article I Section 8 of our bylaws. One third of our shares entitled to vote constitute a quorum for purposes of a meeting of our shareholders.
Dividends
Subject to preferences that may be applicable to any outstanding preferred stock, the holders of our common stock are entitled to receive ratably all dividends, if any, as may be declared from time to time by our Board of Directors out of the funds legally available.
In the event of the liquidation, dissolution or winding up of the Company, the holders of our common stock are entitled to share ratably in all assets remaining after payment of liabilities, subject to prior distribution rights of preferred stock, if any, then outstanding. The common stock has no preemptive or conversion rights. There are no redemption or sinking fund provisions applicable to the common stock. All outstanding shares of common stock are fully paid and non-assessable.
Rights upon Liquidation
Upon our liquidation, dissolution or winding-up, after payment in full of our liabilities and the amounts required to be paid to holders of any outstanding shares of preferred stock, if any, all holders of our common stock, along with the holders of our Series A Convertible Preferred Stock and Series B Convertible Preferred Stock on an “as if” converted basis, will be entitled to receive a pro rata distribution of all of our assets and funds legally available for distribution.
Redemption and Pre-Emptive Rights
No shares of our common stock are subject to redemption or have preemptive rights to purchase additional shares of our common stock or any of our other securities.
Fully Paid and Nonassessable
All of our outstanding shares of common stock are, and the shares of common stock to be issued in this offering will be, fully paid and nonassessable.
| 13 |
Preferred Stock
Our Board of Directors has the authority, without action by our shareholders, to designate and issue up to 50,000,000 shares of preferred stock in one or more series or classes and to designate the rights, preferences and privileges of each series or class, which may be greater than the rights of our common stock. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, redemption rights, liquidation preferences, the number of shares constituting any class or series and the designation of the class or series. Terms selected by our Board of Directors in the future could decrease the amount of earnings and assets available for distribution to holders of shares of common stock or adversely affect the rights and powers, including voting rights, of the holders of shares of common stock without any further vote or action by the stockholders. As a result, the rights of holders of our common stock will be subject to, and may be adversely affected by, the rights of the holders of the Series A Convertible Preferred Stock, and Series B Convertible Preferred Stock or any other preferred stock that may be issued by us in the future, which could have the effect of decreasing the market price of our common stock.
Series A Convertible Preferred Stock
On May 10, 2017 and on July 25, 2017, we issued an aggregate of 12,000,000 shares of convertible preferred stock, designated as the Series A Convertible Preferred Stock pursuant to the certificate of designation and rights filed by us with the Secretary of State of the State of Florida, with an aggregate original purchase price and initial liquidation preference of $3.0 million. Each share of Series A Convertible Preferred Stock was issued for an amount equal to $0.25 per share, which we refer to as the original purchase price. On March 9, 2018 and August 26, 2022, certain holders of Series A Convertible Preferred Stock elected to convert to common stock and, as a result of such conversions, 5,417,000 shares of Series A Preferred remain outstanding.
The following description is a summary of the material provisions of the Series A Convertible Preferred Stock and the certificate of designation and rights and does not purport to be complete. This summary is subject to and is qualified by reference to all the provisions of the Series A Convertible Preferred Stock and certificate of designation and rights of Series A Convertible Preferred Stock, including the definitions of certain terms used in the certificate of designation and rights. We urge you to read this document because it, and not this description, defines the rights of a holder of the Series A Convertible Preferred Stock. A copy of the form of certificate of designation and rights that we filed with the Secretary of State of the State of Florida effective May 10, 2017 as amended and restated effective November 8, 2017 has been incorporated by reference as an exhibit to the registration statement of which this prospectus forms a part.
No Mandatory Redemption Date or Sinking Fund
The shares of Series A Convertible Preferred Stock do not have a mandatory redemption date and are not subject to any sinking fund. The shares of Series A Convertible Preferred Stock will remain outstanding indefinitely unless we elect to redeem them under the circumstances described below in “Redemption” or we otherwise repurchase them or they are converted into shares of our common stock as described below under “Conversion Rights”.
Dividends
The shares of Series A Convertible Preferred Stock are entitled to participate in all dividends declared and paid on shares of company common stock on an “as if” converted basis.
Liquidation Preference
Upon any liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary that is not a Fundamental Transaction (as defined in the certificate of designation), the holders of Series A Convertible Preferred Stock shall be entitled to receive out of the assets, the greater of (i) the product of the number of shares of Series A Preferred Stock then held by such holder, multiplied by the original issue price; and (ii) the amount that would be payable to such holder in the liquidation in respect of Common Stock issuable upon conversion of such shares of Series A Preferred Stock if all outstanding shares of Series A Preferred Stock were converted into Common Stock immediately prior to the Liquidation.
| 14 |
Ranking
The Series A Convertible Preferred Stock ranks (i) on par with the Common Stock and Series B Convertible Preferred Stock and junior to Series C Non-Convertible Preferred Stock as to dividend rights and (ii) on par with Series B Convertible Preferred Stock, junior to Series C Non-Convertible Preferred Stock and senior to Common Stock as to rights upon liquidation, dissolution or winding up of the Company, whether voluntarily or involuntarily.
See “Voting Rights—Matters Requiring Approval of Holders of Series A Convertible Preferred Stock” for a description of the types of issuances of equity securities and other securities of our company requiring approval of holders of a majority of shares of Series A Convertible Preferred Stock then outstanding, voting together as a class.
Redemption
To the extent we have funds legally available therefor, at any time after the fifth anniversary of the original issue date of the Series A Convertible Preferred Stock, we have the right to redeem all or any portion of the outstanding shares of Series A Convertible Preferred Stock at the original issue price of $0.25 by providing at least seventy five (75) days written notice of such redemption to all holders of the then outstanding shares of Series A Convertible Preferred Stock.
Conversion Rights
The holders of shares of Series A Convertible Preferred Stock will, at any time, be entitled to convert some or all of their Series A Convertible Preferred Stock into the number of shares of our common stock obtained by dividing the original purchase price of the shares to be converted by the aggregate Series A conversion price (which originally equaled the original purchase price, but is subject to adjustment), which amount we refer to as the conversion price.
The conversion price will be adjustable upon the occurrence of certain events and transactions to prevent dilution as described under “Adjustments to Conversion Price to Prevent Dilution”. Any shares of our common stock issued upon conversion of the shares of Series A Convertible Preferred Stock shall be validly issued, fully paid and non-assessable. The Company shall in lieu of fractional shares rounded up to the next whole share. The initial conversion price was $0.25 but was adjusted to $2.50 as a result of the Company’s reverse split of 1 for 10 on January 19, 2018 and will be subject to further adjustment following the Company’s contemplated 1 for 60 reverse stock split expected to be effective on January 20, 2023.
Adjustments to Conversion Price to Prevent Dilution
The Series A Convertible Preferred Stock is subject to provisions that protect the holders against dilution by adjustment of the conversion price and/or number of shares of common stock issuable upon conversion in certain events such as a subdivision, combination or reclassification of our outstanding common stock.
Voting Rights—Matters Requiring Approval of Holders of Series A Convertible Preferred Stock
Except as otherwise required by law, the Series A Convertible Preferred Stock shall have no voting rights. However, as long as any shares of Series A Convertible Preferred Stock are outstanding, we shall not, without the affirmative vote of the holders of a majority of the then outstanding shares of the Series A Convertible Preferred Stock, (a) alter or change adversely the powers, preferences or rights given to the Series A Convertible Preferred Stock or alter or amend the certificate of designation, (b) amend its articles of incorporation or other charter documents in any manner that adversely affects any rights of the holders of Series A Convertible Preferred Stock, (c) increase the number of authorized shares of Series A Convertible Preferred Stock, or (d) enter into any agreement with respect to any of the foregoing.
Registration Rights
The holders of the Series A Convertible Preferred Stock were granted certain demand registration rights and piggyback registration rights with respect to the shares of our Common Stock issuable upon conversion of the Series A Preferred Stock and exercise of their associated warrants, subject to customary cutbacks, blackout periods and other exceptions.
| 15 |
Series B Convertible Preferred Stock
On November 8, 2017, we issued 6,600,000 shares of convertible preferred stock, designated as the Series B Convertible Preferred Stock pursuant to the certificate of designation and rights filed by us with the Secretary of State of the State of Florida, with an aggregate original purchase price and initial liquidation preference of $3.3 million. Each share of Series B Convertible Preferred Stock was issued for an amount equal to $0.50 per share, which we refer to as the original purchase price. On August 26, 2022 a certain holder of Series B Convertible Preferred Stock elected to convert to common stock and, as a result of such conversion, 4,050,000 shares of Series B Convertible Preferred Stock remain outstanding.
The following description is a summary of the material provisions of the Series B Convertible Preferred Stock and the certificate of designation and rights and does not purport to be complete. This summary is subject to and is qualified by reference to all the provisions of the Series B Convertible Preferred Stock and certificate of designation and rights of Series B Convertible Preferred Stock, including the definitions of certain terms used in the certificate of designation and rights. We urge you to read this document because it, and not this description, defines the rights of a holder of the Series B Convertible Preferred Stock. A copy of the form of certificate of designation and rights that we filed with the Secretary of State of the State of Florida effective November 8, 2017 has been incorporated by reference as an exhibit to the registration statement of which this prospectus forms a part.
No Mandatory Redemption Date or Sinking Fund
The shares of Series B Convertible Preferred Stock do not have a mandatory redemption date and are not subject to any sinking fund. The shares of Series B Convertible Preferred Stock will remain outstanding indefinitely unless we elect to redeem them under the circumstances described below in “Redemption” or we otherwise repurchase them or they are converted into shares of our common stock as described below under “Conversion Rights”.
Dividends
The shares of Series B Convertible Preferred Stock are entitled to participate in all dividends declared and paid on shares of company common stock on an “as if” converted basis.
Liquidation Preference
Upon any liquidation, dissolution or winding-up of the Company (any such event, a “Liquidation”), whether voluntary or involuntary, each holder of shares of Series B Convertible Preferred Stock shall be entitled to receive, after payment to the Series C Non-Convertible Preferred Stock as provided in the Certificate of Designation of Series C Non-Convertible Preferred Stock, but on par with Series A Convertible Preferred Stock and in preference to the holders of Common Stock, an amount of cash equal to the greater of (i) the product of the number of shares of Series B Convertible Preferred Stock then held by such holder, multiplied by the original issue price; and (ii) the amount that would be payable to such holder in the Liquidation in respect of Common Stock issuable upon conversion of such shares of Series B Convertible Preferred Stock if all outstanding shares of Series B Convertible Preferred Stock were converted into Common Stock immediately prior to the Liquidation (disregarding for this purpose any and all limitations of any kind on such conversion).
Ranking
The Series B Convertible Preferred Stock ranks (i) on par with the Common Stock and Series A Convertible Preferred Stock and junior to Series C Non-Convertible Preferred Stock as to dividend rights and (ii) junior to Series C Non-Convertible Preferred Stock, on par with Series A Convertible Preferred Stock and senior to the Common Stock as to distributions of assets upon liquidation, dissolution or winding up of the Corporation, whether voluntarily or involuntarily.
See “Voting Rights—Matters Requiring Approval of Holders of Series B Convertible Preferred Stock” for a description of the types of issuances of equity securities and other securities of our company requiring approval of holders of a majority of shares of Series B Convertible Preferred Stock then outstanding, voting together as a class.
| 16 |
Redemption
To the extent we have funds legally available therefor, at any time after the fifth anniversary of the original issue date of the Series B Convertible Preferred Stock, we have the right to redeem all or any portion of the outstanding shares of Series B Convertible Preferred Stock at the original issue price of $0.50 by providing at least seventy five (75) days written notice of such redemption to all holders of the then outstanding shares of Series B Convertible Preferred Stock.
Conversion Rights
The holders of shares of Series B Convertible Preferred Stock will, at any time, be entitled to convert some or all of their Series B Convertible Preferred Stock into the number of shares of our common stock obtained by dividing the original purchase price of the shares to be converted by the aggregate Series B conversion price (which originally equaled the original purchase price, but is subject to adjustment), which amount we refer to as the conversion price and then multiplying such product by two (2).
The conversion price will be adjustable upon the occurrence of certain events and transactions to prevent dilution as described under “Adjustments to Conversion Price to Prevent Dilution”. Any shares of our common stock issued upon conversion of the shares of Series B Convertible Preferred Stock shall be validly issued, fully paid and non-assessable. The Company shall either pay cash in lieu of fractional shares or round up to the next whole share. The initial conversion price was $0.50 but was adjusted to $5.00 as a result of the Company’s reverse split of 1 for 10 on January 19, 2018 and will be subject to further adjustment following the Company’s contemplated 1 for 60 reverse stock split expected to be effective on January 20, 2023.
Adjustments to Conversion Price to Prevent Dilution
The Series B Convertible Preferred Stock is subject to provisions that protect the holders against dilution by adjustment of the conversion price and/or number of shares of common stock issuable upon conversion in certain events such as a subdivision, combination or reclassification of our outstanding common stock.
Voting Rights—Matters Requiring Approval of Holders of Series B Convertible Preferred Stock
Except as otherwise required by law, the Series B Convertible Preferred Stock shall have no voting rights. However, as long as any shares of Series B Convertible Preferred Stock are outstanding, we shall not, without the affirmative vote of the holders of a majority of the then outstanding shares of the Series B Convertible Preferred Stock, (a) amend, alter, repeal, restate or supplement (in each case, whether by reclassification, merger, consolidation, reorganization or otherwise) the certificate of designation in any manner that would adversely affect the holders of the Series B Convertible Preferred Stock, (b) authorize or agree to authorize any increase in the number of shares of Series B Convertible Preferred Stock or issue any additional shares of Series B Convertible Preferred Stock, (c) amend, alter or repeal any provision of the Certificate of Incorporation or Bylaws of the Company which would adversely affect any right, preference, privilege or voting power of the Series B Convertible Preferred Stock or the holders thereof or (d) agree to take any of the foregoing actions.
Registration Rights
The holders of the Series B Convertible Preferred Stock were granted certain demand registration rights and piggyback registration rights with respect to the shares of our Common Stock issuable upon conversion of the Series B Preferred Stock and exercise of their associated warrants, subject to customary cutbacks, blackout periods and other exceptions.
Series C Non-Voting, Non-Convertible Preferred Stock
On November 8, 2017, we issued to a single older 100 shares of non-convertible preferred stock, designated as the Series C Non-Voting, Non-Convertible Preferred Stock pursuant to the certificate of designation and rights filed by us with the Secretary of State of the State of Florida, with a stated value and liquidation preference equal to $33,847.9874 per share, which we refer to as the Stated Value. The shares of Series C Non-Voting, Non-Convertible Preferred Stock were entitled to payment-in-kind (“PIK”) dividends thereon at the annual rate of twelve percent (12%) (the “Initial Rate”) of its Stated Value, payable by issuing additional shares of Series C Non-Voting, Non-Convertible Preferred Stock within thirty days after the end of each calendar year, pro-rata for partial years. During the three months ended March 31, 2021, the Company provided a notice of redemption, to the holder of the Company’s Series C Preferred Stock to redeem all outstanding Series C Preferred Stock (which included the dividend of 26.697 shares paid on January 28, 2021 and any accrued dividends due through the redemption date of March 13, 2021). The Series C Preferred Stock redemption amount of approximately $5.6 million was paid on March 15, 2021 and all outstanding shares of Series C Preferred Stock were cancelled.
| 17 |
Series D Preferred Stock-Converted to Common Stock
On July 13, 2018, our board of directors designated 9,364,000 shares of our preferred stock as Series D Convertible Preferred Stock (“Series D Preferred Stock”), which were subsequently issued on July 17, 2018, none of which are currently issued and outstanding. The preferences and rights of the Series D Preferred Stock was set forth in a Certificate of Designation (the “Series D Certificate of Designation”). Pursuant to a transfer agency agreement between us and Continental Stock Transfer & Trust Company, as transfer agent, the Series D Preferred Stock was issued in book-entry form and represented only by one or more global certificates deposited with The Depository Trust Company, or DTC, and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC. Prior to the end of 2018, all of 9,364,000 shares of Series D Preferred Stock had converted to common stock and as such, the Company no longer has any Series D Preferred Stock outstanding.
Registration Rights
Series A Preferred Stock Private Placement. Pursuant to the May 10, 2017 Registration Rights Agreement, we granted certain demand registration rights and piggyback registration rights with respect to the shares of our Common Stock issuable upon conversion of the Series A Preferred Stock and the exercise of the common stock warrants that were issued commensurate with the issuance of the Series A Preferred Stock.
Series B Preferred Stock Private Placement. Pursuant to the November 8, 2017 Amended and Restated Registration Right Agreement, we granted certain demand registration rights and piggyback registration rights with respect to the shares of our Common Stock issuable upon conversion of the Series B Preferred Stock and the exercise of the common stock warrants that were issued commensurate with the issuance of the Series B Preferred Stock.. The Amended and Restated Registration Rights Agreement amended the previous registration rights agreement entered into in connection with our Series A Preferred Stock Financing in May 2017.
The following descriptions are summaries of the material terms that are included in our amended and restated articles of incorporation (as amended) and our bylaws (as amended) as well as the specific agreements such descriptions relate to. This summary is qualified in its entirety by the specific terms and provisions contained in our restated articles of incorporation, bylaws and the specific agreements described herein, copies of which we have filed as exhibits to the registration statement of which this prospectus is a part, and by the provisions of applicable law.
Certain Anti-Takeover Provisions
Florida Law
We are not subject to the statutory anti-takeover provisions under Florida law because in our articles of incorporation we have specifically elected to opt out of both the “control-share acquisitions” (F.S. 607.0902) and the “affiliated transactions” (F.S. 607.0901) statutes. Since these anti-takeover statutes do not apply to a corporation that has specifically elected to opt out of such provisions, we would not be able to invoke the protection of such statutes in the event of a hostile takeover attempt.
Articles of Incorporation and Bylaw Provisions
Our articles of incorporation and bylaws contain provisions that could have an anti-takeover effect. These provisions include
| ● | authorization of the issuance of “blank check” preferred stock that could be issued by our Board of Directors without shareholder approval and that may be substantially dilutive or contain preferences or rights objectionable to an acquiror; |
| ● | the ability of the Board of Directors to amend the bylaws without shareholder approval; |
| ● | vacancies on our board may only be filled by the remaining Directors and not our shareholders; and |
| ● | requirements that only our Board, our President or holders of more than 10% of our shares can call a special meeting of shareholders. |
These provisions in our articles of incorporation and bylaws could delay or discourage transactions involving an actual or potential change in control of us, including transactions in which shareholders might otherwise receive a premium for their shares over their current prices. Such provisions could also limit the ability of shareholders to approve transactions that shareholders may deem to be in their best interests and could adversely affect the price of our common stock.
Listing of Common Stock
Our common stock is currently listed on the NYSE American under the trading symbol “OGEN”.
Transfer Agent and Registrar
The transfer agent and registrar of our common stock is Continental Stock Transfer & Trust Company, 1 State Street 30th Floor, New York, New York 10004, telephone: (212) 509-4000.
| 18 |
The following description, together with the additional information that we include in any applicable prospectus supplement and in any related free writing prospectus that we may authorize to be distributed to you, summarizes the material terms and provisions of the warrants that we may offer under this prospectus, which may be issued in one or more series. Warrants may be offered independently or in combination with other securities offered by any prospectus supplement. While the terms we have summarized below will apply generally to any warrants that we may offer under this prospectus, we will describe the particular terms of any series of warrants in more detail in the applicable prospectus supplement. The following description of warrants will apply to the warrants offered by this prospectus unless we provide otherwise in the applicable prospectus supplement. The applicable prospectus supplement for a particular series of warrants may specify different or additional terms.
Any warrants issued under this prospectus may be evidenced by warrant certificates. Warrants also may be issued under an applicable warrant agreement that we enter into with a warrant agent. We will indicate the name and address of the warrant agent, if applicable, in the prospectus supplement relating to the particular series of warrants being offered.
We will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from reports that we file with the SEC, the form of warrant and/or the warrant agreement and warrant certificate, as applicable, that contain the terms of the particular series of warrants we are offering, and any supplemental agreements, before the issuance of such warrants. The following description summarizes the material terms and provisions of the warrants and is subject to, and qualified in its entirety by reference to, all the provisions of the form of warrant and/or the warrant agreement and warrant certificate, as applicable, and any supplemental agreements applicable to a particular series of warrants that we may offer under this prospectus. We urge you to read the applicable prospectus supplement related to the particular series of warrants that we may offer under this prospectus, as well as any related free writing prospectuses, and the complete form of warrant and/or the warrant agreement and warrant certificate, as applicable, and any supplemental agreements, that contain the terms of the warrants.
General
We will describe in the applicable prospectus supplement the terms of the series of warrants being offered, including:
| ● | the title of such securities; |
| ● | the offering price and aggregate number of warrants offered; |
| ● | the currency or currencies for which the warrants may be purchased; |
| ● | if applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each such security or each principal amount of such security; |
| ● | if applicable, the date on and after which the warrants and the related securities will be separately transferable; |
| ● | if applicable, the minimum or maximum amount of such warrants which may be exercise at any one time; |
| ● | in the case of warrants to purchase common stock, the number of shares of common stock, purchasable upon the exercise of one warrant and the price at which, and the currency in which, these shares may be purchased upon such exercise; |
| ● | the effect of any merger, consolidation, sale or other disposition of our business on the warrant agreements and the warrants; |
| ● | the dates on which the right to exercise the warrants shall commence or expire; |
| ● | the terms of any rights to redeem or call the warrants; |
| ● | the terms of any rights to force the exercise of the warrants; |
| 19 |
| ● | any provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the warrants; |
| ● | the dates on which the right to exercise the warrants will commence and expire; |
| ● | the manner in which the warrant agreements and warrants may be modified; |
| ● | a discussion of any material or special U.S. federal income tax considerations of holding or exercising the warrants; |
| ● | the antidilution provisions of the warrant, if any; |
| ● | the terms of the securities issuable upon exercise of the warrants; and |
| ● | any other specific terms, preferences, rights or limitations of or restrictions on the warrants. |
Before exercising their warrants, holders of warrants will not have any of the rights of holders of the securities purchasable upon such exercise, including: in the case of warrants to purchase common stock, the right to receive dividends, if any, or, payments upon our liquidation, dissolution or winding up or to exercise voting rights, if any.
Exercise of Warrants
Each warrant will entitle the holder to purchase the securities that we specify in the applicable prospectus supplement at the exercise price that we describe in the applicable prospectus supplement. The warrants may be exercised as set forth in the prospectus supplement relating to the warrants offered. Unless we otherwise specify in the applicable prospectus supplement, warrants may be exercised at any time up to the close of business on the expiration date set forth in the prospectus supplement relating to the warrants offered thereby. After the close of business on the expiration date, unexercised warrants will become void.
Unless we otherwise specify in the applicable prospectus supplement, holders of the warrants may exercise the warrants by delivering the warrant certificate representing the warrants to be exercised together with specified information, and paying the required amount to the warrant agent in immediately available funds, as provided in the applicable prospectus supplement. We will set forth on the reverse side of the warrant certificate and in the applicable prospectus supplement the information that the holder of the warrant will be required to deliver to the warrant agent in connection with the exercise of the warrant.
Upon receipt of payment and the warrant or warrant certificate, as applicable, properly completed and duly executed at the corporate trust office of the warrant agent, if any, or any other office, including ours, indicated in the prospectus supplement, we will, as soon as practicable, issue and deliver the securities purchasable upon such exercise. If less than all of the warrants (or the warrants represented by such warrant certificate) are exercised, a new warrant or a new warrant certificate, as applicable, will be issued for the remaining warrants.
Governing Law
Unless we otherwise specify in the applicable prospectus supplement, the warrants and any warrant agreements will be governed by and construed in accordance with the laws of the State of New York.
Enforceability of Rights by Holders of Warrants
Each warrant agent, if any, will act solely as our agent under the applicable warrant agreement and will not assume any obligation or relationship of agency or trust with any holder of any warrant. A single bank or trust company may act as warrant agent for more than one issue of warrants. A warrant agent will have no duty or responsibility in case of any default by us under the applicable warrant agreement or warrant, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a warrant may, without the consent of the related warrant agent or the holder of any other warrant, enforce by appropriate legal action its right to exercise, and receive the securities purchasable upon exercise of, its warrants.
| 20 |
Units
We may issue units consisting of any combination of our common stock and warrants. We will issue each unit so that the holder of the unit is also the holder of each security included in the unit. As a result, the holder of a unit will have the rights and obligations of a holder of each included security. The unit agreement under which a unit is issued may provide that the securities included in the unit may not be held or transferred separately, at any time or at any time before a specified date.
The summary below and that contained in any prospectus supplement is qualified in its entirety by reference to all of the provisions of the unit agreement and/or unit certificate, and depositary arrangements, if applicable. We urge you to read the applicable prospectus supplements and any related free writing prospectuses related to the units that we may offer under this prospectus, as well as the complete unit agreement and/or unit certificate, and depositary arrangements, as applicable, that contain the terms of the units.
We will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from reports that we file with the SEC, the form of unit agreement and/or unit certificate, and depositary arrangements, as applicable, that contain the terms of the particular series of units we are offering, and any supplemental agreements, before the issuance of such units.
The applicable prospectus supplement, information incorporated by reference or free writing prospectus may describe:
| ● | the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately; |
| ● | any provisions for the issuance, payment, settlement, transfer, or exchange of the units or of the securities composing the units; |
| ● | whether the units will be issued in fully registered or global form; and |
| ● | any other terms of the units. |
The applicable provisions described in this section, as well as those described under “Common Stock” and “Warrants” above, will apply to each unit and to each security included in each unit, respectively
| 21 |
We can issue securities in registered form or in the form of one or more global securities. We describe global securities in greater detail below. We refer to those persons who have securities registered in their own names on the books that we or any applicable trustee, depositary or warrant agent maintain for this purpose as the “holders” of those securities. These persons are the legal holders of the securities. We refer to those persons who, indirectly through others, own beneficial interests in securities that are not registered in their own names, as “indirect holders” of those securities. As we discuss below, indirect holders are not legal holders, and investors in securities issued in book-entry form or in street name will be indirect holders.
Book-Entry Holders
We may issue securities in book-entry form only, as we will specify in the applicable prospectus supplement. This means securities may be represented by one or more global securities registered in the name of a financial institution that holds them as depositary on behalf of other financial institutions that participate in the depositary’s book-entry system. These participating institutions, which are referred to as participants, in turn, hold beneficial interests in the securities on behalf of themselves or their customers.
Only the person in whose name a security is registered is recognized as the holder of that security. Global securities will be registered in the name of the depositary or its participants. Consequently, for global securities, we will recognize only the depositary as the holder of the securities, and we will make all payments on the securities to the depositary. The depositary passes along the payments it receives to its participants, which in turn pass the payments along to their customers who are the beneficial owners. The depositary and its participants do so under agreements they have made with one another or with their customers; they are not obligated to do so under the terms of the securities.
As a result, investors in a global security will not own securities directly. Instead, they will own beneficial interests in a global security, through a bank, broker or other financial institution that participates in the depositary’s book-entry system or holds an interest through a participant. As long as the securities are issued in global form, investors will be indirect holders, and not legal holders, of the securities.
Street Name Holders
We may terminate a global security or issue securities that are not issued in global form. In these cases, investors may choose to hold their securities in their own names or in “street name”. Securities held by an investor in street name would be registered in the name of a bank, broker or other financial institution that the investor chooses, and the investor would hold only a beneficial interest in those securities through an account he or she maintains at that institution.
For securities held in street name, we or any applicable trustee or depositary will recognize only the intermediary banks, brokers and other financial institutions in whose names the securities are registered as the holders of those securities, and we or any such trustee or depositary will make all payments on those securities to them. These institutions pass along the payments they receive to their customers who are the beneficial owners, but only because they agree to do so in their customer agreements or because they are legally required to do so. Investors who hold securities in street name will be indirect holders, not legal holders, of those securities.
Legal Holders
Our obligations, as well as the obligations of any applicable trustee or third party employed by us or a trustee, run only to the legal holders of the securities. We do not have obligations to investors who hold beneficial interests in global securities, in street name or by any other indirect means. This will be the case whether an investor chooses to be an indirect holder of a security or has no choice because we are issuing the securities only in global form.
For example, once we make a payment or give a notice to the holder, we have no further responsibility for the payment or notice even if that holder is required, under agreements with its participants or customers or by law, to pass it along to the indirect holders but does not do so. Similarly, we may want to obtain the approval of the holders to amend an indenture, to relieve us of the consequences of a default or of our obligation to comply with a particular provision of an indenture, or for other purposes. In such an event, we would seek approval only from the holders, and not the indirect holders, of the securities. Whether and how the legal holders contact the indirect holders is up to the legal holders.
| 22 |
Special Considerations for Indirect Holders
If you hold securities through a bank, broker or other financial institution, either in book-entry form because the securities are represented by one or more global securities or in street name, you should check with your own institution to find out:
| ● | how it handles securities payments and notices; | |
| ● | whether it imposes fees or charges; | |
| ● | how it would handle a request for the holders’ consent, if ever required; | |
| ● | whether and how you can instruct it to send you securities registered in your own name so you can be a holder, if that is permitted in the future; | |
| ● | how it would exercise rights under the securities if there were a default or other event triggering the need for holders to act to protect their interests; and | |
| ● | if the securities are in book-entry form, how the depositary’s rules and procedures will affect these matters. |
Global Securities
A global security is a security that represents one or any other number of individual securities held by a depositary. Generally, all securities represented by the same global securities will have the same terms.
Each security issued in book-entry form will be represented by a global security that we issue to, deposit with and register in the name of a financial institution or its nominee that we select. The financial institution that we select for this purpose is called the depositary. Unless we specify otherwise in the applicable prospectus supplement, The Depository Trust Company, New York, New York, known as DTC, will be the depositary for all securities issued in book-entry form.
A global security may not be transferred to or registered in the name of anyone other than the depositary, its nominee or a successor depositary, unless special termination situations arise. We describe those situations below under “—Special Situations When a Global Security Will Be Terminated”. As a result of these arrangements, the depositary, or its nominee, will be the sole registered owner and legal holder of all securities represented by a global security, and investors will be permitted to own only beneficial interests in a global security. Beneficial interests must be held by means of an account with a broker, bank or other financial institution that in turn has an account with the depositary or with another institution that does. Thus, an investor whose security is represented by a global security will not be a legal holder of the security, but only an indirect holder of a beneficial interest in the global security.
If the prospectus supplement for a particular security indicates that the security will be issued as a global security, then the security will be represented by a global security at all times unless and until the global security is terminated. If termination occurs, we may issue the securities through another book-entry clearing system or decide that the securities may no longer be held through any book-entry clearing system.
Special Considerations for Global Securities
As an indirect holder, an investor’s rights relating to a global security will be governed by the account rules of the investor’s financial institution and of the depositary, as well as general laws relating to securities transfers. We do not recognize an indirect holder as a holder of securities and instead deal only with the depositary that holds the global security.
| 23 |
If securities are issued only as global securities, an investor should be aware of the following:
| ● | an investor cannot cause the securities to be registered in his or her name, and cannot obtain non-global certificates for his or her interest in the securities, except in the special situations we describe below; | |
| ● | an investor will be an indirect holder and must look to his or her own bank or broker for payments on the securities and protection of his or her legal rights relating to the securities, as we describe above; | |
| ● | an investor may not be able to sell interests in the securities to some insurance companies and to other institutions that are required by law to own their securities in non-book-entry form; | |
| ● | an investor may not be able to pledge his or her interest in the global security in circumstances where certificates representing the securities must be delivered to the lender or other beneficiary of the pledge in order for the pledge to be effective; | |
| ● | the depositary’s policies, which may change from time to time, will govern payments, transfers, exchanges and other matters relating to an investor’s interest in the global security; | |
| ● | we and any applicable trustee have no responsibility for any aspect of the depositary’s actions or for its records of ownership interests in the global security, nor will we or any applicable trustee supervise the depositary in any way; | |
| ● | the depositary may, and we understand that DTC will, require that those who purchase and sell interests in the global security within its book-entry system use immediately available funds, and your broker or bank may require you to do so as well; and | |
| ● | financial institutions that participate in the depositary’s book-entry system, and through which an investor holds its interest in the global security, may also have their own policies affecting payments, notices and other matters relating to the securities. |
There may be more than one financial intermediary in the chain of ownership for an investor. We do not monitor and are not responsible for the actions of any of those intermediaries.
Special Situations When a Global Security Will Be Terminated
In a few special situations described below, a global security will terminate and interests in it will be exchanged for physical certificates representing those interests. After that exchange, the choice of whether to hold securities directly or in street name will be up to the investor. Investors must consult their own banks or brokers to find out how to have their interests in securities transferred to their own names, so that they will be direct holders. We have described the rights of holders and street name investors above.
Unless we provide otherwise in the applicable prospectus supplement, a global security will terminate when the following special situations occur:
| ● | if the depositary notifies us that it is unwilling, unable or no longer qualified to continue as depositary for that global security and we do not appoint another institution to act as depositary within 90 days; | |
| ● | if we notify any applicable trustee that we wish to terminate that global security; or | |
| ● | if an event of default has occurred with regard to securities represented by that global security and has not been cured or waived. |
The applicable prospectus supplement may also list additional situations for terminating a global security that would apply only to the particular series of securities covered by the prospectus supplement. When a global security terminates, the depositary, and neither we nor any applicable trustee, is responsible for deciding the names of the institutions that will be the initial direct holders.
| 24 |
We may sell the securities from time to time pursuant to underwritten public offerings, direct sales to the public, direct sales to the public, negotiated transactions, block trades or a combination of these methods. We may sell the securities to or through underwriters or dealers, through one or more agents, or directly to one or more purchasers. We may distribute securities from time to time in one or more transactions:
| ● | at a fixed price or prices, which may be changed; |
| ● | at market prices prevailing at the time of sale; |
| ● | at prices related to such prevailing market prices; |
| ● | at varying prices determined at the time of sale; or |
| ● | at negotiated prices. |
We may also sell equity securities covered by this registration statement in an “at the market” offering as defined in Rule 415(a)(4) under the Securities Act. Such offering may be made into an existing trading market for such securities in transactions at other than a fixed price on or through the facilities of NYSE American or any other securities exchange or quotation or trading service on which such securities may be listed, quoted or traded at the time of sale. Such at the market offerings, if any, may be conducted by underwriters acting as principal or agent.
A prospectus supplement or (and any related free writing prospectus that we may authorize to be provided to you) will describe the terms of the offering of the securities, including, to the extent applicable:
| ● | the name or names of any underwriters, dealers or agents, if any; |
| ● | the purchase price of the securities and the proceeds we will receive from the sale; |
| ● | any over-allotment options under which underwriters may purchase additional securities from us; |
| ● | any agency fees or underwriting discounts and other items constituting agents’ or underwriters’ compensation; |
| ● | any public offering price; |
| ● | any discounts or concessions allowed or reallowed or paid to dealers; and |
| ● | any securities exchange or market on which the securities may be listed. |
Only the agents or underwriters named in each prospectus supplement will be agents or underwriters in connection with the securities offered by a prospectus supplement.
Offers to purchase the securities being offered by this prospectus may be solicited directly. Agents may also be designated to solicit offers to purchase the securities from time to time. Any agent involved in the offer or sale of our securities will be identified in a prospectus supplement. Unless the prospectus supplement states otherwise, our agent will act on a best-efforts basis for the period of its appointment.
If a dealer is utilized in the sale of the securities being offered by this prospectus, the securities will be sold to the dealer, as principal. The dealer may then resell the securities to the public at varying prices to be determined by the dealer at the time of resale.
If an underwriter is utilized in the sale of the securities being offered by this prospectus, an underwriting agreement will be executed with the underwriter at the time of sale and the name of any underwriter will be provided in the prospectus supplement that the underwriter will use to make resales of the securities to the public. In connection with the sale of the securities, we, or the purchasers of securities for whom the underwriter may act as agent, may compensate the underwriter in the form of underwriting discounts or commissions. The underwriter may sell the securities to or through dealers, and those dealers may receive compensation in the form of discounts, concessions or commissions from the underwriters and/or commissions from the purchasers for which they may act as agent. Unless otherwise indicated in a prospectus supplement, an agent will be acting on a best efforts basis and a dealer will purchase securities as a principal, and may then resell the securities at varying prices to be determined by the dealer.
| 25 |
Any compensation paid to underwriters, dealers or agents in connection with the offering of the securities, and any discounts, concessions or commissions allowed by underwriters to participating dealers will be provided in the applicable prospectus supplement. Underwriters, dealers and agents participating in the distribution of the securities may be deemed to be underwriters within the meaning of the Securities Act, and any discounts and commissions received by them and any profit realized by them on resale of the securities may be deemed to be underwriting discounts and commissions. We may enter into agreements to indemnify underwriters, dealers and agents against civil liabilities, including liabilities under the Securities Act, or to contribute to payments they may be required to make in respect thereof and to reimburse those persons for certain expenses.
Any common stock will be listed on the NYSE American, but any other securities may or may not be listed on a national securities exchange. To facilitate the offering of securities, certain persons participating in the offering may engage in transactions that stabilize, maintain or otherwise affect the price of the securities. This may include over-allotments or short sales of the securities, which involve the sale by persons participating in the offering of more securities than were sold to them. In these circumstances, these persons would cover such over-allotments or short positions by making purchases in the open market or by exercising their over-allotment option, if any. In addition, these persons may stabilize or maintain the price of the securities by bidding for or purchasing securities in the open market or by imposing penalty bids, whereby selling concessions allowed to dealers participating in the offering may be reclaimed if securities sold by them are repurchased in connection with stabilization transactions. The effect of these transactions may be to stabilize or maintain the market price of the securities at a level above that which might otherwise prevail in the open market. These transactions may be discontinued at any time.
We may authorize underwriters, dealers or other persons acting as our agents to solicit offers by certain institutions or other suitable purchasers to purchase securities from us at the public offering price set forth in the prospectus supplement, pursuant to delayed delivery contracts providing for payment and delivery on the date stated in each applicable prospectus supplement. Each contract will be for an amount not less than, and the aggregate amount of securities sold pursuant to such contracts shall not be less nor more than, the respective amounts stated in each applicable prospectus supplement. Institutions with whom the contracts, when authorized, may be made include commercial and savings banks, insurance companies, pension funds, investment companies, educational and charitable institutions and other institutions, but shall in all cases be subject to our approval. Delayed delivery contracts will be subject only to those conditions set forth in each applicable prospectus supplement and include the condition that the purchase of the securities covered by the delayed delivery contracts will not at the time of delivery be prohibited under the laws of any jurisdiction in the United States to which the purchaser is subject. Each prospectus supplement will set forth any commissions we pay for solicitation of these contracts. The underwriters and agents will not have any responsibility with respect to the validity or performance of these contracts.
All securities we may offer, other than common stock, will be new issues of securities with no established trading market. Any agents or underwriters may make a market in these securities, but will not be obligated to do so and may discontinue any market making at any time without notice. We cannot guarantee the liquidity of the trading markets for any securities. There is currently no market for any of the offered securities, other than our common stock which is listed on the on the NYSE American. Any common stock will be listed on the NYSE American but any other securities may or may not be listed on a national securities exchange. We have no current plans for listing of the, warrants on any securities exchange or quotation system; any such listing with respect to any particular warrants will be described in the applicable prospectus supplement or other offering materials, as the case may be.
Any agents and underwriters who are qualified market makers on the NYSE American may engage in passive market making transactions in the securities on the NYSE American in accordance with Regulation M, during the business day prior to the pricing of the offering, before the commencement of offers or sales of the securities. Passive market makers must comply with applicable volume and price limitations and must be identified as passive market makers. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for such security; if all independent bids are lowered below the passive market maker’s bid, however, the passive market maker’s bid must then be lowered when certain purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
In addition, we may enter into derivative transactions with third parties, or sell securities not covered by this prospectus to third parties in privately negotiated transactions. If the applicable prospectus supplement so indicates, in connection with those derivatives, the third parties may sell securities covered by this prospectus and the applicable prospectus supplement, including in short sale transactions. If so, the third party may use securities pledged by us or borrowed from us or others to settle those sales or to close out any related open borrowings of stock, and may use securities received from us in settlement of those derivatives to close out any related open borrowings of stock. The third party in such sale transactions will be an underwriter and, if not identified in this prospectus, will be named in the applicable prospectus supplement (or a post-effective amendment). In addition, we may otherwise loan or pledge securities to a financial institution or other third party that in turn may sell the securities short using this prospectus and an applicable prospectus supplement. Such financial institution or other third party may transfer its economic short position to investors in our securities or in connection with a concurrent offering of other securities.
The specific terms of any lock-up provisions in respect of any given offering will be described in the applicable prospectus supplement.
The underwriters, dealers and agents may engage in transactions with us, or perform services for us, in the ordinary course of business for which they receive compensation.
In compliance with guidelines of the Financial Industry Regulatory Authority, Inc., or FINRA, the maximum compensation to be received by any FINRA member or independent broker dealer may not exceed 8% of the aggregate amount of the securities offered pursuant to this prospectus and any applicable prospectus supplement.
| 26 |
Unless otherwise indicated in the applicable prospectus supplement, certain legal matters in connection with the offering and the validity of the securities offered by this prospectus, and any supplement thereto, will be passed upon for us by Shumaker, Loop & Kendrick, LLP. Additional legal matters may be passed upon for us or any underwriters, dealers or agents, by counsel that we will name in the applicable prospectus supplement.
| 27 |
The audited financial statements of Oragenics, Inc. as of December 31, 2021 and 2020, and for the years ended December 31, 2021 and 2020, as set forth in its report included in our Annual Report on Form 10-K for the year ended December 31, 2021, incorporated by reference in this prospectus have been audited by Mayer Hoffman McCann P.C., an independent registered public accounting firm, as stated in their report dated March 24, 2022, which is incorporated by reference herein, and has been so incorporated in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
| 28 |
WHERE YOU CAN FIND ADDITIONAL INFORMATION
This prospectus is part of a registration statement we filed with the SEC. This prospectus does not contain all of the information set forth in the registration statement and the exhibits to the registration statement. For further information with respect to us and the securities we are offering under this prospectus, we refer you to the registration statement and the exhibits and schedules filed as a part of the registration statement. You should rely only on the information contained in this prospectus or incorporated by reference in this prospectus. We have not authorized anyone else to provide you with different information. We are not making an offer of these securities in any state where the offer is not permitted. You should not assume that the information in this prospectus is accurate as of any date other than the date on the front page of this prospectus, regardless of the time of delivery of this prospectus or any sale of the securities offered by this prospectus.
We file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public at the SEC’s website at http://www.sec.gov.
Copies of certain information filed by us with the SEC are also available on our website at www.Oragenics.com Information contained in or accessible through our website does not constitute a part of this prospectus and is not incorporated by reference in this prospectus. We have included our website address as an inactive textual reference only.
| 29 |
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
The SEC allows us to incorporate by reference the information we file with it, which means that we can disclose important information to you by referring you to another document that we have filed separately with the SEC. You should read the information incorporated by reference because it is an important part of this prospectus. We incorporate by reference the following information or documents that we have filed with the SEC, excluding any portions of any Current Report on Form 8-K that are not deemed “filed” pursuant to the General Instructions of Form 8-K:
| ● | Our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the SEC on March 24, 2022 and our Form 10-K/A for the year ended December 31, 2021, filed with the SEC on July 29, 2022; |
| ● | Our Quarterly Reports on Form 10-Q for the quarter ended March 31, 2022, filed with the SEC on May 13, 2022, for the quarter ended June 30, 2022 filed with the SEC on August 9, 2022 and for the quarter ended September 30, 2022 filed with the SEC on November 14, 2022; |
| ● | Our Definitive Proxy Statement on Schedule 14A, filed with the SEC on October 31, 2022; |
| ● | Our Current Reports on Form 8-K, filed January 26, 2022, February 28, 2022, March 10, 2022, April 6, 2022, April 19, 2022, May 17, 2022, June 23, 2022, July 8, 2022, August 3, 2022, August 24, 2022, September 30, 2022, October 3, 2022, November 16, 2022, December 15, 2022, December 19, 2022, December 20, 2022, December 22, 2022 and December 23, 2022; |
| ● | The description of our common stock set forth in our registration statement on Form 8-A12B, filed April 8, 2013, including any amendments or reports filed for purposes of updating such description. |
Any information in any of the foregoing documents will automatically be deemed to be modified or superseded to the extent that information in this prospectus or in a later filed document that is incorporated or deemed to be incorporated herein by reference modifies or replaces such information.
We also incorporate by reference into this prospectus all documents (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items) that are filed by us with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act (i) after the date of the initial filing of the registration statement of which this prospectus forms a part and prior to effectiveness of the registration statement, or (ii) after the date of this prospectus but prior to the termination of the offering. These documents include periodic reports, such as Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as well as proxy statements.
We will provide to each person, including any beneficial owner, to whom a prospectus is delivered, without charge upon written or oral request, a copy of any or all of the documents that are incorporated by reference into this prospectus but not delivered with the prospectus, including exhibits which are specifically incorporated by reference into such documents. You may request a copy of these filings at no cost, by writing to or telephoning us at the following address: Oragenics, Inc., 4902 Eisenhower Boulevard, Suite 125, Tampa, Florida 33634, Attention: Corporate Secretary.
Any statement contained in this prospectus or contained in a document incorporated or deemed to be incorporated by reference into this prospectus will be deemed to be modified or superseded to the extent that a statement contained in this prospectus or any subsequently filed supplement to this prospectus, or document deemed to be incorporated by reference into this prospectus, modifies or supersedes such statement.
| 30 |
Shares of Common Stock
Pre-Funded Warrants to Purchase Common Stock

PROSPECTUS SUPPLEMENT
ThinkEquity
, 2024